��Ŀ����
11��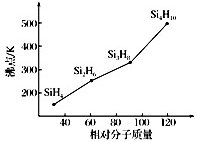 ̫���ܵ����ͨ�����ЧӦ���߹⻯ѧЧӦֱ�Ӱѹ���ת���ɵ��ܵ�װ�ã�����ϳ������裬����ͭ�������Ȼ����
̫���ܵ����ͨ�����ЧӦ���߹⻯ѧЧӦֱ�Ӱѹ���ת���ɵ��ܵ�װ�ã�����ϳ������裬����ͭ�������Ȼ������1���صĻ�̬ԭ�ӵĵ����Ų�ʽ��1s22s22p63s23p63d104s24p1����[Ar]3d104s24p1����
��2����Ϊ��4����Ԫ�أ����ڵ�Ԫ��������壬��3��Ԫ�صĵ�һ�����ܴӴ�С˳��ΪBr��As��Se����Ԫ�ط��ű�ʾ����
��3����̬SeO3���ӵ����幹��Ϊƽ�������Σ�
��4�����飨SinH2n+2���ķе�������Է��������ı仯��ϵ��ͼ��ʾ���������ֱ仯��ϵ��ԭ���ǣ��������Է�������Խ���Ӽ䷶�»���Խǿ��
��5������Ԫ�ش���ͬһ�������Ԫ�ؾ���ȱ�����ԣ��仯�����������мӺ��ԣ�������ᣨH3BO3����ˮ��Һ������ˮ��Ӧ����[B��OH��4]-������һԪ��������ʣ���[B��OH��4]-��B��ԭ���ӻ�����Ϊsp3��
��6������Cu�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ����ͭ�������ӵ���Һ����÷�Ӧ�����ӷ���ʽΪCu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
��7��һ��ͭ��Ͻ�����������������ܶѻ��Ľṹ���ھ����У�Auԭ��λ�ڶ��㣬Cuԭ��λ�����ģ���úϽ���Auԭ����Cuԭ�Ӹ���֮��Ϊ1��3�����þ����ı߳�Ϊapm����Ͻ���ܶ�Ϊ$\frac{��197+64��3����1{0}^{30}}{{a}^{3}{N}_{A}}$��g•cm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
���� ��1������31��Ԫ�أ�����ԭ�Ӻ�������Ų����ɿ���д�������Ų�ʽ��
��2���顢����������Ԫ�ض��ǵ�4���ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ�����Ԫ��ԭ��4p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������Br��As��Se���ݴ˴��⣻
��3����̬SeO3����������ԭ�ӵļ۲���Ӷ��������жϷ��ӹ��ͣ�
��4�����飨SinH2n+2�����Ƿ��Ӿ��壬���Ӿ���ķе�ߵ�ȡ���ڷ��Ӽ��������������Ӽ�����������Է��������Ĵ�С�йأ��ݴ˴��⣻
��5�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
��6������������ԭ��Ӧ��Ԫ�غ͵���غ㣬��д�����ӷ���ʽ��
��7�����þ�̯���������ֽ���ԭ�Ӹ���֮�ȣ����ݦ�=$\frac{m}{V}$���㣮
��� �⣺��1������31��Ԫ�أ�����ԭ�Ӻ�������Ų����ɿ���д�������Ų�ʽΪ��1s22s22p63s23p63d104s24p1����[Ar]3d104s24p1����
�ʴ�Ϊ��1s22s22p63s23p63d104s24p1����[Ar]3d104s24p1����
��2���顢����������Ԫ�ض��ǵ�4���ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ�����Ԫ��ԭ��4p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������Br��As��Se���ʴ�Ϊ��Br��As��Se��
��3����̬SeO3����������ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+0}{2}$=3���µ��Ӷԣ����Է��ӹ���Ϊƽ�������Σ��ʴ�Ϊ��ƽ�������Σ�
��4�����飨SinH2n+2�����Ƿ��Ӿ��壬���Ӿ���ķе�ߵ�ȡ���ڷ��Ӽ��������������Ӽ�����������Է��������Ĵ�С�йأ��������Է�������Խ���Ӽ䷶�»���Խǿ���ʴ�Ϊ���������Է�������Խ���Ӽ䷶�»���Խǿ��
��5��[B��OH��4]-��B�ļ۲���Ӷ�=4+$\frac{1}{2}$��3+1-4��1��=4�����Բ�ȡsp3�ӻ����ʴ�Ϊ��sp3��
��6������Cu�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ��˵�������ܻ���ٽ������������ʹ�ͬ���õĽ�������й�������Ϊ������������Cu2+�γ������ӣ�������ٽ�ʹ��Ӧ���У�����ʽ�ɱ�ʾΪ��Cu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
�ʴ�Ϊ��Cu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
��7���ھ����У�Auԭ��λ�ڶ��㣬Cuԭ��λ�����ģ��þ�����Auԭ�Ӹ���=8��$\frac{1}{8}$=1��Cuԭ�Ӹ���=6��$\frac{1}{2}$=3�����ԸúϽ���Auԭ����Cuԭ�Ӹ���֮��=1��3��
�������V=��a��10-10cm��3��ÿ��������ͭԭ�Ӹ�����3��Auԭ�Ӹ�����1�����=$\frac{\frac{197+64��3}{{N}_{A}}}{��a��1{0}^{-10}��^{3}}$=$\frac{��197+64��3����1{0}^{30}}{{a}^{3}{N}_{A}}$g•cm-3��
�ʴ�Ϊ��1��3��$\frac{��197+64��3����1{0}^{30}}{{a}^{3}{N}_{A}}$��
���� ������Ҫ�����˺�������Ų�����һ�����ܡ����ӿռ乹�͡��ӻ���ʽ�������ܶȵļ��㣬�Ѷ��еȣ�����ʱҪע��Ի���֪ʶ��������ã�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 0.1mol•L-1��NaOH��Һ��K+��Na+��SO42-��CO32- | |
| B�� | 0.1mol•L-1��Na2CO3��Һ��K+��Ba2+��NO3-��Cl- | |
| C�� | 0.1mol•L-1FeCl3��Һ��K+��NH4+��I-��SCN- | |
| D�� | 0.1mol•L-1��HCl��Һ��Ca2+��Na+��ClO-��NO3- |
| A�� | ����������ԭ | B�� | ������������ | C�� | ����������ԭ | D�� | ������������ |
| A�� | 1 mol/L NaCl��Һ | B�� | 2 mol/L NH4Cl��Һ | ||
| C�� | 1.5 mol/L MgCl2��Һ | D�� | 2 mol/L AlCl3��Һ |
| A�� | 5.6 g ��������������ȼ�գ�ת�Ƶ�����Ϊ0.2 NA | |
| B�� | 1mol H3O+�����ĵ�����Ϊ11NA | |
| C�� | 71 g��������������Ӧ�õ��ĵ�����һ��Ϊ2NA | |
| D�� | 16 g O2��O3�Ļ�������У���O2������Ϊ0.5NA |
| A�� | ����C���� | B�� | �������������Сһ�� | ||
| C�� | ����������䣬����H2O��g�� | D�� | ����ѹǿ���䣬����N2 |
 ���������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������£�ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵������ȷ���ǣ�������
���������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������£�ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵������ȷ���ǣ�������| A�� | ��������FeCl3��Һ����ɫ | |
| B�� | ��������KMnO4��Һ����ɫ��ȥ����֤����ṹ�д���̼̼˫�� | |
| C�� | 1mol�����ʷֱ���Ũ��ˮ��H2��Ӧʱ�������Br2��H2�ֱ�Ϊ4mol��7mol | |
| D�� | �÷����е�����̼ԭ�Ӳ����ܹ�ƽ�� |