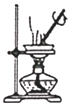
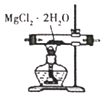
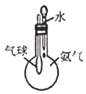
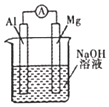
��Ŀ����
����Ŀ����֪������±�����ڴ����������¿��������������±���⣬C�������г������л���ұ��ܱ����Ը��������Һ����Ϊ�����ᡣ��������֮���ת����ϵ��ͼ��ʾ(���������������������ȥ)��

��ش��������⣺
(1)д�����ʵĽṹ��ʽ��A____________��C___________��E____________��
(2)�ڢ�����6����Ӧ�У�������ȥ��Ӧ����________(���ţ���ͬ)������������Ӧ����________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
��_____________________________________________________��
��____________________________________________________��
��_____________________________________________________��
H��I_________________________________________________��
���𰸡�CH2==CH2 CH3CH2OH ![]() �ڢ� �� CH3CH2Cl��
�ڢ� �� CH3CH2Cl��![]()
![]() CH3CH2OH
CH3CH2OH ![]() CH2==CH2����H2O
CH2==CH2����H2O ![]() �� NaCl �� H2O
�� NaCl �� H2O 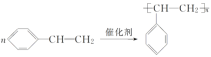
��������
D��Cl2�ڹ�������������G��GΪ±������G�ڼ�����������NaOH��Һ����ȡ����Ӧ����![]() ����GΪ
����GΪ![]() ��G������ȥ��Ӧ���ɵ�HΪ
��G������ȥ��Ӧ���ɵ�HΪ![]() ��H�����Ӿ۷�Ӧ���ɵĸ߾���IΪ
��H�����Ӿ۷�Ӧ���ɵĸ߾���IΪ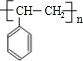 �����ƿ�֪DΪ
�����ƿ�֪DΪ![]() ��D����������Ӧ���ɵ�EΪ
��D����������Ӧ���ɵ�EΪ![]() ����A��B���ת����ϵ��֪BΪ±��������B�뱽������Ӧ����D����BΪCH3CH2Cl����AΪCH2=CH2��CΪCH3CH2OH��E��C����������Ӧ���ɵ�FΪ
����A��B���ת����ϵ��֪BΪ±��������B�뱽������Ӧ����D����BΪCH3CH2Cl����AΪCH2=CH2��CΪCH3CH2OH��E��C����������Ӧ���ɵ�FΪ![]() ��
��
��1��ͨ�����Ϸ���֪��A��C��E�ṹ��ʽ�ֱ�ΪCH2�TCH2��CH3CH2OH��![]() ��
��
�ʴ�Ϊ��CH2�TCH2��CH3CH2OH��![]() ��
��
��2���ڢ�����6����Ӧ�У�������ȥ��Ӧ���Ǣڢ���CΪ����EΪ�ᣬ��E��C����������Ӧ�����ѡ�����ʴ�Ϊ���ڢ�������
��3����Ϊ������ͱ���ȡ����Ӧ����Ӧ����ʽΪ�� ![]() ��
��
��Ϊ�Ҵ�����ȥ��Ӧ����Ӧ����ʽΪ��CH3CH2OH ![]() CH2==CH2����H2O��
CH2==CH2����H2O��
��Ӧ����ʽΪ��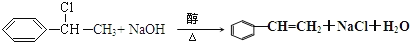 ��
��
H��I��Ӧ����ʽΪ�� ![]()
![]()
 ��
��
�ʴ�Ϊ��![]() �� CH3CH2OH
�� CH3CH2OH ![]() CH2==CH2����H2O ��
CH2==CH2����H2O �� ��
�� ![]()
![]()
 ��
��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���������ף�����Ũ����ͻ��ý�����Ӧ�����У���������Ũ�ȵĽ��ͣ������ɵIJ�������4����2����3�۵��Ļ����
��FeSO4 + NO![]() Fe(NO)SO4(��ɫ)����H��0��
Fe(NO)SO4(��ɫ)����H��0��
��NO2��NO���ܱ�����KMnO4��Һ�������ա�
�װ�����ͼ��ʾ��ʵ��װ�ý���ʵ�飺
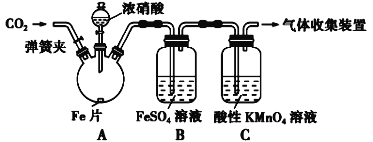
ʵ������������¼�����ʾ��
ʵ����� | ʵ������ |
���ɼУ�ͨ��һ��ʱ��CO2���رյ��ɼ� | |
��Һ©����������Ũ���Ỻ��������ƿ�У��رջ��� | ���������� |
������ƿ����Ӧ��ʼ��ֹͣ���� | ��A���к���ɫ���������һ��ʱ���������ɫ��dz��B����Һ����ɫ��C����Һ��ɫ��dz�� �ڷ�Ӧֹͣ��A������ʣ�� |
��ش��������⣺
�� ����ǰ������ƿ�е���Ũ����û�����������ԭ����________________________________
�� �����Ƿ����ɣ�3�۵��Ļ����Ӧ���е�ʵ�������________________________________
�� ��ȡ����B����Һ�����ȣ�ʵ��������____________________________________________�����û�ѧƽ��ԭ������ԭ��________________________________________________________�������ݸ�����ó����ۣ��������ᷴӦ��NO���ɡ�
������Ϊ�ó�A����NO���ɵ�֤�ݲ��㡣Ϊ��ȡ�����֤�ݣ����Բ��ø�װ�úͲ������ж���ʵ�飬�������ĸı���____________________________��֤����NO���ɵ�ʵ��������________________________________________________