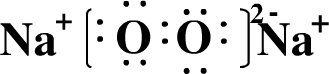��Ŀ����
���ֶ�����Ԫ�ص����ʻ�ṹ��Ϣ���±����������Ϣ�ش��������⣺
��1��C�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ
��2��Ԫ��A��ԭ�Ӻ�����
��3��D�������������Dԭ�ӵ��ӻ�������
��4��B���Ȼ���ľ���������
��5��A��ÿ��˫ԭ�ӷ����к���
| Ԫ�ط��� | Ԫ�����ʻ�ṹ��Ϣ |
| A | ԭ�Ӻ���L��δ�ɶԵ�������� |
| B | ԭ�Ӱ뾶�Ƕ���������Ԫ�������� |
| C | �ؿ��к������Ľ���Ԫ�� |
| D | ԭ�Ӻ��������ֲ�ͬ�����ĵ��ӣ����������2�ԳɶԵ��� |
3s23p1
3s23p1
��2��Ԫ��A��ԭ�Ӻ�����
2
2
����״��ͬ��ԭ�ӹ������ԭ������㹲��5
5
�ֲ�ͬ�˶�״̬�ĵ��ӣ���3��D�������������Dԭ�ӵ��ӻ�������
sp2
sp2
�����ӵĿռ乹��Ϊƽ����������
ƽ����������
����4��B���Ȼ���ľ���������
���Ӿ���
���Ӿ���
������ͬ�����������Ԫ�ص��Ȼ�����ȣ��۵�ϸߵ���NaCl
NaCl
���ѧʽ����������NaCl��CsCl�������Ӿ��壬��NaCl�ľ����ܴ���CsCl
NaCl��CsCl�������Ӿ��壬��NaCl�ľ����ܴ���CsCl
����5��A��ÿ��˫ԭ�ӷ����к���
1����
1����
����2����
2����
�������以Ϊ�ȵ��������Ļ�ѧʽΪCO��CN-��C22-��
CO��CN-��C22-��
������д2������������Aԭ�Ӻ���L��δ�ɶԵ�������࣬�����Ų�ʽΪ1S22S22P3��ӦΪNԪ�أ�
Bԭ�Ӱ뾶�Ƕ���������Ԫ�������ģ�ӦΪNaԪ�أ�
C�ؿ��к������Ľ���Ԫ�أ�ΪAlԪ�أ�
Dԭ�Ӻ��������ֲ�ͬ�����ĵ��ӣ����������2�ԳɶԵ��ӣ������Ų�ʽΪ1S22S22P63S23P4��ӦΪSԪ�أ�
��1��CΪAlԪ�أ������Ų�ʽΪ1S22s23s23p1���Դ��ж�̬ԭ�ӵļ۵����Ų�ʽ��
��2��A�ĵ����Ų�ʽΪ1S22S22P3��ԭ�Ӻ��������κͷĴ���������״��ͬ�Ĺ�����������5�����ӣ����ӵ��˶�״̬������ͬ��
��3��D�����������ΪSO3����3���ļ����µ��Ӷ���Ϊ��
= 0����۲���Ӷ�Ϊ3���Դ��ж��ӻ����ͺͷ��ӵ����幹�ͣ�
��4��B���Ȼ���ΪNaCl���������Ӿ��壬����Ӱ�쾧���ܵ����رȽ϶��ߵ��۷е�ߵͣ�
��5��A��ÿ��˫ԭ�ӷ�����ԭ�Ӽ�Ļ�ѧ��ΪN��N���Դ��жϻ�ѧ�������Լ��ȵ����壮
Bԭ�Ӱ뾶�Ƕ���������Ԫ�������ģ�ӦΪNaԪ�أ�
C�ؿ��к������Ľ���Ԫ�أ�ΪAlԪ�أ�
Dԭ�Ӻ��������ֲ�ͬ�����ĵ��ӣ����������2�ԳɶԵ��ӣ������Ų�ʽΪ1S22S22P63S23P4��ӦΪSԪ�أ�
��1��CΪAlԪ�أ������Ų�ʽΪ1S22s23s23p1���Դ��ж�̬ԭ�ӵļ۵����Ų�ʽ��
��2��A�ĵ����Ų�ʽΪ1S22S22P3��ԭ�Ӻ��������κͷĴ���������״��ͬ�Ĺ�����������5�����ӣ����ӵ��˶�״̬������ͬ��
��3��D�����������ΪSO3����3���ļ����µ��Ӷ���Ϊ��
| 6-3��2 |
| 2 |
��4��B���Ȼ���ΪNaCl���������Ӿ��壬����Ӱ�쾧���ܵ����رȽ϶��ߵ��۷е�ߵͣ�
��5��A��ÿ��˫ԭ�ӷ�����ԭ�Ӽ�Ļ�ѧ��ΪN��N���Դ��жϻ�ѧ�������Լ��ȵ����壮
����⣺��1��CΪAlԪ�أ������Ų�ʽΪ1S22s23s23p1����̬ԭ�ӵļ۵����Ų�ʽΪ 3s23p1���ʴ�Ϊ��3s23p1��
��2��A�ĵ����Ų�ʽΪ1S22S22P3��ԭ�Ӻ��������κͷĴ���������״��ͬ�Ĺ�����������5�����ӣ����ӵ��˶�״̬������ͬ���ʴ�Ϊ��2��5��
��3��D�����������ΪSO3����3���ļ����µ��Ӷ���Ϊ��
= 0����۲���Ӷ�Ϊ3���ӻ�����Ϊsp2�ӻ������ӵ����幹��Ϊƽ���������Σ��ʴ�Ϊ��sp2��ƽ���������Σ�
��4��B���Ȼ���ΪNaCl���������Ӿ��壬Ӱ�����Ӿ����۷е�ߵ͵������Ǿ����ܵĴ�С�����Ӱ뾶ԽС��������Խ��NaCl�ľ����ܴ���CsCl��
�ʴ�Ϊ�����Ӿ��壻NaCl��NaCl��CsCl�������Ӿ��壬��NaCl�ľ����ܴ���CsCl��
��5��A��ÿ��˫ԭ�ӷ�����ԭ�Ӽ�Ļ�ѧ��ΪN��N������1���ļ���2���м�����������14�����ӣ����以Ϊ�ȵ���������CO��CN-��C22-�ȣ�
�ʴ�Ϊ��1���ģ� 2���У�CO��CN-��C22-�ȣ�
��2��A�ĵ����Ų�ʽΪ1S22S22P3��ԭ�Ӻ��������κͷĴ���������״��ͬ�Ĺ�����������5�����ӣ����ӵ��˶�״̬������ͬ���ʴ�Ϊ��2��5��
��3��D�����������ΪSO3����3���ļ����µ��Ӷ���Ϊ��
| 6-3��2 |
| 2 |
��4��B���Ȼ���ΪNaCl���������Ӿ��壬Ӱ�����Ӿ����۷е�ߵ͵������Ǿ����ܵĴ�С�����Ӱ뾶ԽС��������Խ��NaCl�ľ����ܴ���CsCl��
�ʴ�Ϊ�����Ӿ��壻NaCl��NaCl��CsCl�������Ӿ��壬��NaCl�ľ����ܴ���CsCl��
��5��A��ÿ��˫ԭ�ӷ�����ԭ�Ӽ�Ļ�ѧ��ΪN��N������1���ļ���2���м�����������14�����ӣ����以Ϊ�ȵ���������CO��CN-��C22-�ȣ�
�ʴ�Ϊ��1���ģ� 2���У�CO��CN-��C22-�ȣ�
���������⿼��Ԫ���ƶ��⣬��Ŀ�ѶȽϴ��ƶ�Ԫ�ص�����Ϊ����Ĺؼ���ע�����֪ʶ�Ļ��ۣ�����ԭ�ӽṹ����ع��ɣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ