��Ŀ����
�̷�(FeSO4��7H2O)���ᷨ����һ��ϡ�н�����Ʒ�����в����ĸ���Ʒ����Ʒ���Ϊ����ɫ����ɫ�ᾧ���塣���������ɵ��ڼ���ˮ�е�pH����ˮ���������л���ϣ������ٳ�������ҪӦ����ˮ�ʾ�����ҵ��ˮ������ͬʱ����ɱ�����á�
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ (��>��<��=")40%" ��
��2��ʵ��������20%��������(100�˷������ẬSO3 20��)����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL(��״��)����ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200 mL 2 mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H��=6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H��=3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H��= NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS���������� (������λС��)��
��1��7.14 mol��L��1 ; ��(��2��) ��2��0.77(2��)
��3��FeSO4��Fe2(SO4)3��10H2O (3��)����4����42��60mL(3��)����0.33��1/3(3��)
���������������1���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ��
c�� =7.14 mol��L��1��
=7.14 mol��L��1��
50%��������30%����������(�����ʻ�Ϊ1���ܶȷֱ��Ǧ�1�ͦ�2)��ϣ�������Ũ��Ϊ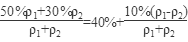 ��40%��
��40%��
��2����Ϊ20%��������Ϊ80��H2SO4��SO3 20�ˣ�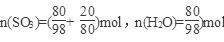 ��
��
����SO3��nH2O��ʾ20%�ķ������ᣬ��n=0.77��
��3��������Һ����������BaCl2��Һ�����˵ó���9.32�ˣ�n(SO42-)=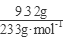 =0.04mol����ͨ��112mL(��״��)����ǡ�ý�Fe2����ȫ����(2Fe2��+Cl2==2Fe3++2Cl-)��n(Fe2+)=2��
=0.04mol����ͨ��112mL(��״��)����ǡ�ý�Fe2����ȫ����(2Fe2��+Cl2==2Fe3++2Cl-)��n(Fe2+)=2��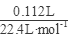 =0.01mol���ɵ���غ�n(Fe3+)=0.02mol�� 7.32�˾��庬��0.01molFeSO4Ϊ1.52g��0.01mol Fe2(SO4)3Ϊ4.00g������H2OΪ1.80g��0.1mol������Ļ�ѧʽFeSO4��Fe2(SO4)3��10H2O��
=0.01mol���ɵ���غ�n(Fe3+)=0.02mol�� 7.32�˾��庬��0.01molFeSO4Ϊ1.52g��0.01mol Fe2(SO4)3Ϊ4.00g������H2OΪ1.80g��0.1mol������Ļ�ѧʽFeSO4��Fe2(SO4)3��10H2O��
��4�������������ȫ��ΪCuS����n��CuS��=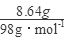 =0.09mol����Ҫ��������ʵ���Ϊy��
=0.09mol����Ҫ��������ʵ���Ϊy��
8NO3-+3CuS + 8H+==3Cu2++3SO42-+8NO��+4H2O��
3 8
0.09mol y
y=0.24mol
ʣ�����������ʵ���Ϊ��0.4mol-0.24mol=0.16mol��
0.112mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
NO3-+3Fe2++4H+==NO��+3Fe3++2H2O
3mol 4
1��10-3VL��2mol/L 0.16mol�����V=60��
����Vֵ��ΧΪ��42��V��60��
����V=48����48mL��NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��Ҫ��������ʵ���Ϊ��
NO3-+3Fe2+ + 4H+��NO��+3Fe3++2H2O
3mol 4
0.048L��2mol/L n
��ã�n=0.128mol��
����������ﷴӦ����������ʵ���Ϊ��0.4mol-0.128mol=0.272mol��
��Cu2S�����ʵ���xmol��CuS�����ʵ���Ϊymol��160x+96y=8.64g�٣�
10NO3-+3Cu2S+16H+��6Cu2++10NO��+3SO42-+8H2O
3 16
x 16x/3
8NO3-+3CuS+8H+��3Cu2++3SO42-+8NO��+4H2O��
3 8
y 8y/3
16x/3+8y/3=0.272��
�ɢ٢ڽ�ã�x��0.036; y��0.03
�������CuS������������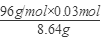 ��100%��33.33%��
��100%��33.33%��
���㣺��ѧ�뼼�������������Ũ�ȱ仯�ص㡢ͭ�����������ᷴӦ�ļ��㡣

 ���Ͱ�ͨ��ĩ���ϵ�д�
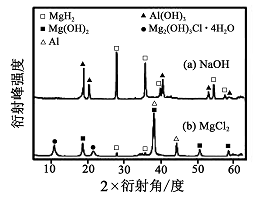
���Ͱ�ͨ��ĩ���ϵ�д��ú���A12O3��SiO2������FeO��xFe2O3�������Ʊ�A12(SO4)3��18H2O�������������£�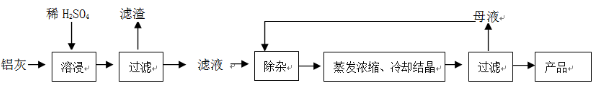
��һ�������£�MnO4- ����Mn2+��Ӧ����MnO2��
��֪�� �����������������pH
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 2.7 |
| ��ȫ����ʱ | 5.2 | 9.7 | 3.2 |
��2��������Һ�л�����Fe2���ķ����� (ע���Լ�������)��
��3�������ӡ����������¼�������:
��������Һ�м������KMnO4��Һ��������Һ��pHΪ3.2��
�����ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ:
������MnSO4���Ϻ�ɫ��ʧ�����ˡ�
�ٲ�����Ŀ�� ��������Һ��pHΪ3.2��Ŀ���� ��
�����ij����м���ŨHCl�����ȣ���˵�������д���MnO2�������� ��д���䷴Ӧ����ʽ ��
�ۢ��м���MnSO4��Ŀ���� ��
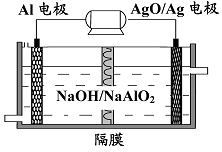
��4���Ӷ��ѭ��ʹ�ú�ĸҺ�пɻ��յ���Ҫ������ ���ѧʽ����
������Ʒ�������п�����������������Ʒ�ı�������һ�����ܵ�Fe3O4��ijѧϰС��Ϊ���о�������Ƭ���ֱ����������ʵ�������
�ٰ�һ����������Ƭ�ӹ��ɾ��ȷ�ĩ��
��ȡm g�÷�ĩ������28.00 mL 1 mol��L�������У�ǡ����ȫ��Ӧ�����ɱ�״���µ�����134.4 mL������Һ�е���KSCN��Һ������������
����ȡ���ݲ�ͬ�����ķ�ĩ���ּӼӵ���ͬ���(V)�����ʵ���Ũ�Ⱦ�Ϊl0.00 mol��L������������Һ�У���ַ�Ӧ����ȫ���ܽ⣬�йص�ʵ���������±���ʾ(����NO�������Ψһ��ԭ����)��
| ʵ����� | �� | �� | �� |
| �����ĩ����/g | 13.68 | 27.36 | 34.20 |
| ����������������״����/L | 2.912 | 5.824 | 6.720 |
������и��⣺
��1��ʵ���������Һ�е������� (д��ѧʽ)����Ʒ��n(Fe)��n(Fe3O4)= ��m�� ��
��2������ʵ�����ÿ��������Һ�����(V)��mL����
��3������ʵ���������Һ�м�������ͭ�ۣ�Ҫʹ��Һ��Cu2ʮ��Fe2+��Fe3+ͬʱ���ڣ������ͭ�۵����ʵ����ķ�Χ��
��16�֣�ͭ��Ũ���ᷴӦ��ʵ��װ����ͼ��ʾ��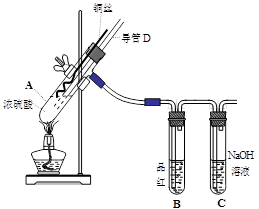
��1�� װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ�� ����Ӧ�������Թ�B�е������� ���Թ�C�������� ��
��2�� ����D���¶�(���߶�)Ӧλ�� ��Һ���ϡ�Һ���£�������D�������У���ʵ��������ų�װ���е�SO2���� ��
ʵ���з����Թ��ڳ��˲�����ɫ�����⣬��ͭ˿���滹�к�ɫ��������ɣ����п��ܺ���CuO��Cu2O��CuS��Cu2S��Ϊ̽���ijɷ֣����������µ�ʵ�顣
�������Ͽ�֪��Cu2O + 2HCl =CuCl2+ Cu + H2O�� 2Cu2O + O2���� 4CuO��2CuS+3O2����2CuO+2SO2��Cu2S+2O2����2CuO+SO2��CuS�� Cu2S��ϡHCl����Ӧ��
|
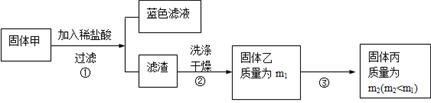 ��3�� �������ڿ���������ʱ��ʹ�õ�ʵ���������˲����������żܡ��ƾ����⣬�������У� ��
��3�� �������ڿ���������ʱ��ʹ�õ�ʵ���������˲����������żܡ��ƾ����⣬�������У� ����4�� ���չ����У���������Ӧ�⣬�����ܷ�����Ӧ�ķ���ʽΪ ��
��5�����ۣ�������CuO�����϶����е������� ��
������������Ҫ�������Σ���ũҵ������ũҩ����Ҫ��С����벡���������������ݼ����ڹ�ҵ������Ⱦɫ����������īˮ��ľ�ķ����ȡ�
��1�����Ƶ��̷���FeSO4��7H2O����dz��ɫ�ģ����ڿ����м��ױ�ɻ�ɫ������ɫ�ļ�ʽ������[Fe(OH)SO4]��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2����֪FeSO4�ڲ�ͬ�����·ֽ�õ����ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��
SO3�۵���16.8�棬�е���44.8�档
ij�о���ѧϰС����������װ�ý���ʵ��̽�����ڼ���������FeSO4�ķֽ�����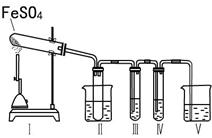
����װ�â�͢����������������Իش��������⣺
�٢�װ���ձ���ˮ���¶�Ӧ������ ��ѡ�0�桢25�桢50�桱����װ�â�������� ��
��װ�â��е��Լ������� ��ѡ����ţ���ͬ���������� ����֤����������к���SO2�� װ�â��е��Լ������� ��
| A��2 mol/LNa2CO3��Һ |
| B��Ʒ����Һ |
| C��0.5 mol/LBaCl2��Һ |
| D��0.5 mol/LBa(NO3)2 |
F. ���۵⻯����Һ
��װ��V���Լ�ΪNaOH��Һ��������Ӧ�����ӷ���ʽΪ ��
��Ϊ�˼���������ɷ֣�ȡ��Ӧ��Ĺ������Թ��У���ϡ�����ܽ⣬��������Һ�ֳ����ݣ�������
��ʵ�飺
| �������� | Ԥ��ʵ������ | Ԥ��ʵ����� |
| ������һ����Һ�м��� �� | | �����к���Fe2O3 |
| ����һ����Һ�еμ�2�λ�ɫK3[Fe(CN)6]��Һ�� | ������ɫ���� | |
������22.8 g FeSO4������ʵ�飬��ȫ�ֽ�õ�11.2 g���壬����Fe2O3����������=
����ȷ��0.1%��
 2Cu+SO2��Ӧ��ȡ��ͭ������32gCu2S����ʱ������ת����Ŀ�� ��
2Cu+SO2��Ӧ��ȡ��ͭ������32gCu2S����ʱ������ת����Ŀ�� ��