��Ŀ����
����Ŀ���о����ʵ���ɡ��ṹ�����ڸ��õ��������ʵ����ʡ�

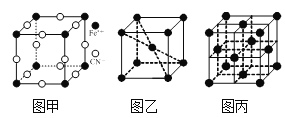
��ͼ3��As4S4���ӵĽṹ���÷����к��Ǽ��Թ��ۼ�����Ŀ��_____����̬Asԭ�ӵ���Χ�����Ų�ʽ��______��
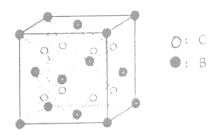
��ͼ1��ij�־���ľ������þ���Ļ�ѧʽ��______��
��ͼ1��Ӧ������ͼ3��Ӧ�����۵�ϸߵ���______��ԭ����______��
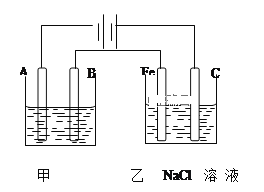
��NaCl���۵�Ϊ801.3�棬MgO���۵�ߴ�2800�档MgO�۵�ߵ�ԭ����______��
��ͼ3��ʾ�����ᾧ��IJ�״�ṹ������H3BO3����ͨ�����������H3BO3����Bԭ�ӵĹ���ӻ���ʽ��______��1 mol H3BO3�����к��е������Ŀ��_____��
�ʽ��ʯ������衢���ɰ��SiC���ľ���������ͬ�����ǵ��۵��ɸߵ��͵�˳����_____��
���𰸡�2 4s24p3 SiO2 SiO2 SiO2��ԭ�Ӿ��壬���۵���ڷ��Ӿ���As4S4�� �������Ӿ��壬��MgO�����ĵ�ɶ࣬���Ӱ뾶֮��С�������ܴ��۵�� sp2 3��6.02��1023 ���ʯ�����ɰ�������
��������
��1��ͼ3��As4S4���ӵĽṹ���÷����к��Ǽ��Թ��ۼ�����Ŀ��2����̬Asԭ������� ������Ϊ5����Χ�����Ų�ʽ��4s24p3��
��2��Si��8��1/8+6��1/2+4=8,O:4��4=16���ʻ�ѧʽΪSiO2��
��3��ͼ1��Ӧ����SiO2��ԭ�Ӿ��壬ͼ3��Ӧ������As4S4���ӣ��Ƿ��Ӿ��壬�۵�ϸߵ���SiO2��
��4��MgO�۵�ߵ�ԭ���ǣ��������Ӿ��壬��MgO�����ĵ�ɶ࣬���Ӱ뾶֮��С�������ܴ��۵�ߣ�
��5��ͼ3��ʾ�����ᾧ��IJ�״�ṹ������H3BO3����ͨ�����������B��O�γ������������µ��Ӷԣ�H3BO3����Bԭ�ӵĹ���ӻ���ʽ��sp2��
һ��H3BO3���Ӷ�Ӧ��6�������һ�������Ӧ��2��H3BO3���ӣ���˺���1 molH3BO3���ӵľ�������3mol�����
��6�����ʯ�������ͽ��ɰ��̼���裩������ԭ�Ӿ��壬ԭ�Ӱ뾶ԽС�����ۼ��ļ���Խ�̣����ۼ�Խ�ȶ���������۵�Խ�ߣ�������Si-Si��Si-C��C-C���������۷е㣺���ʯ�����ɰ������衣

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д� ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д�����Ŀ���±��������ʲ��ܰ���ͼ����������ʾһ����ɣ���ϵ�ת������
ѡ�� | A | B | C | D |
|
a | Na | Al | Fe | Cu | |
b | NaOH | Al2O3 | FeCl3 | CuSO4 | |
c | NaCl | Al��OH��3 | FeCl2 | CuCl2 |
A.AB.BC.CD.D
����Ŀ��һ�������£�ͨ�����з�Ӧ�����Ʊ������մɵ�ԭ��MgO�� MgSO3(s) + CO(g)![]() MgO(s) + CO2(g) +SO2(g) ��H>0���÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ�������
MgO(s) + CO2(g) +SO2(g) ��H>0���÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ�������

ѡ�� | x | y |
A | �¶� | �����ڻ��������ܶ� |
B | CO�����ʵ��� | CO2��CO�����ʵ���֮�� |
C | SO2��Ũ�� | ƽ�ⳣ��K |
D | MgSO4����������������� | CO��ת���� |