��Ŀ����
����Ŀ��������ⷨ���Ѱ۲����ķ�Һ[���д���![]() ������
������![]() ��
��![]() ]����������Ͳ�Ѫ�������������������������£�
]����������Ͳ�Ѫ�������������������������£�
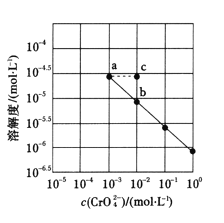
��֪��![]() ������ˮ����ˮ�п��Ե���Ϊ
������ˮ����ˮ�п��Ե���Ϊ![]() ��
��![]() ˮ���
ˮ���![]() ����Ϊ���淴Ӧ��������������Ļ�ѧʽΪ��
����Ϊ���淴Ӧ��������������Ļ�ѧʽΪ��![]() ����ش�
����ش�
��1������ټ�����м��Ŀ��һ�ǻ�ԭ����![]() ������ʹ����
������ʹ����![]() ת��Ϊ
ת��Ϊ![]() ��������ƽ���ƶ���ԭ�����͵õ�������ԭ��________��
��������ƽ���ƶ���ԭ�����͵õ�������ԭ��________��
��2�����������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ________��
��3������ܵ����ӷ���ʽ��______��
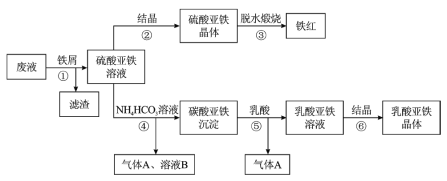
��4�������������Һ���������______��________���ˡ�ϴ�ӡ�����������������塣�þ�����ʱӦ____________��
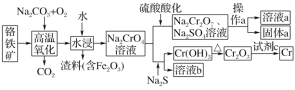
���𰸡�TiOSO4+(x+1)H2OTiO2xH2O��+H2SO4��TiO2++(x+1)H2OTiO2xH2O��+2H+����м��H+��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O���� 1��4 Fe2++2HCO3-�TFeCO3��+H2O+CO2�� ����Ũ�� ��ȴ�ᾧ �ܷⱣ��
��������
�����̿�֪������мʱ��Fe��H2SO4������Fe2(SO4)3��Ӧ������FeSO4��TiOSO4ˮ������TiO2xH2O�����ˣ�����ΪTiO2xH2O��Fe����ҺΪFeSO4��FeSO4��Һͨ������Ũ������ȴ�ᾧ������ϴ�ӵõ������������壬��ˮ�����յõ���������FeSO4��Һ�м�̼�������Һ������Fe2++2HCO3-�TFeCO3��+H2O+CO2�������˷����̼�������������ټ������ܽ���������������Һ�Ͷ�����̼����֪AΪCO2����ҺB������泥�����������Һͨ������Ũ������ȴ�ᾧ������ϴ�ӵõ������������壬�ݴ˷������
(1)TiOSO4+(x+1)H2OTiO2xH2O��+H2SO4��TiO2++(x+1)H2OTiO2xH2O��+2H+����м��H+��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O��������˼�����м�ܹ�ʹ����TiOSO4ת��ΪTiO2xH2O������ �ʴ�Ϊ��TiOSO4+(x+1)H2OTiO2xH2O��+H2SO4��TiO2++(x+1)H2OTiO2xH2O��+2H+����м��H+��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O������
(2)���������ڿ��������������������������4FeSO4+O2 ![]() 2Fe2O3+4SO3����������O2����ԭ����FeSO4�������������ͻ�ԭ�������ʵ���֮��Ϊ1��4���ʴ�Ϊ��1��4��
2Fe2O3+4SO3����������O2����ԭ����FeSO4�������������ͻ�ԭ�������ʵ���֮��Ϊ1��4���ʴ�Ϊ��1��4��
(3)���������������������FeSO4��̼�������Һ��Ӧ����̼����������������狀Ͷ�����̼���壬��Ӧ�����ӷ���ʽ��ΪFe2++2HCO3-�TFeCO3��+H2O+CO2�����ʴ�Ϊ��Fe2++2HCO3-�TFeCO3��+H2O+CO2����
(4)�����������Һ���������������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����������������壬�þ�����ʱӦ�ܷⱣ�棬��ֹ���������ʴ�Ϊ������Ũ������ȴ�ᾧ���ܷⱣ�档