��Ŀ����
��17�֣�����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɡ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��
|
�������� |
Fe(OH)3 |
Al(OH)3 |
Mg(OH)2 |
|
��ʼ����pH |
1.5 |
3.3 |
9.4 |
ʵ�鲽�����£�
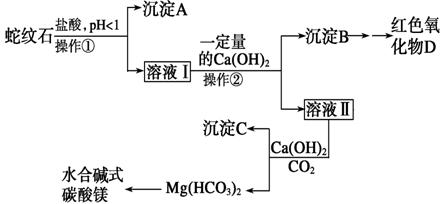
��ش��������⣺
(1)ʵ������ɲ��������õ��IJ���������________________ ��
(2)������Һ�����Ƿ���Fe3���IJ����������___________________________
_____________________________________________________________________��
(3)�ӳ��������B����ȡD��Ӧ����һ���Լ����з��룬�䷴Ӧ�����ӷ���ʽΪ________________________________________________________________________��
�ٽ���__________�� �� (������дʵ���������)��
(4)��������Ӧ������ҺpH�ĺ�����Χ��____(�����)��
A����1.5 B��1.5��3.3
C��7��8 D������9.4
(5)Ϊ̽�����õ�ˮ�ϼ�ʽ̼��þ[xMgCO3��yMg(OH)2��zH2O]����ɣ�ȡ��7.28 g��װ��A�IJ������У��밴����D����˳������װ�����Ӻ�(����ţ�װ�ÿ��ظ�ʹ��)��________________________________________________________________________��
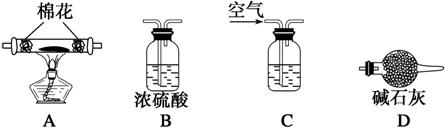
װ��CӦʢ���Լ���______________________��
��17�֣�(������ҳ244ҳ15��)
(1)��������©�����ձ� ��3�֣���һ��1�֣�
(2)ȡ������Һ�����Թ��У�����KSCN��Һ������������˵��Fe3��������ȫ������Һ���ɫ��˵��Fe3��û�г�����ȫ ��3�֣�
(3)OH����Al(OH)3===AlO��2H2O����3�֣� ���ˡ�ϴ�ӡ����ȣ�3�֣�
(4)C ��1�֣�
(5)C�D��B�D��A�D��B�D��D�D��B(��D) ��3�֣���
NaOH��Һ�����������𰸣�1�֣�
����������

 ��У����ϵ�д�
��У����ϵ�д���17�֣�����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɡ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
ʵ�鲽�����£�
��ش��������⣺
(1)ʵ������ɲ��������õ��IJ���������________________ ��
(2)������Һ�����Ƿ���Fe3���IJ����������___________________________
_____________________________________________________________________��
(3)�ӳ��������B����ȡD��Ӧ����һ���Լ����з��룬�䷴Ӧ�����ӷ���ʽΪ________________________________________________________________________��
�ٽ���__________�� �� (������дʵ���������)��
(4)��������Ӧ������ҺpH�ĺ�����Χ��____(�����)��
A����1.5 B��1.5��3.3
C��7��8 D������9.4
(5)Ϊ̽�����õ�ˮ�ϼ�ʽ̼��þ[xMgCO3��yMg(OH)2��zH2O]����ɣ�ȡ��7.28 g��װ��A�IJ������У��밴����D����˳������װ�����Ӻ�(����ţ�װ�ÿ��ظ�ʹ��)��________________________________________________________________________��
װ��CӦʢ���Լ���______________________��


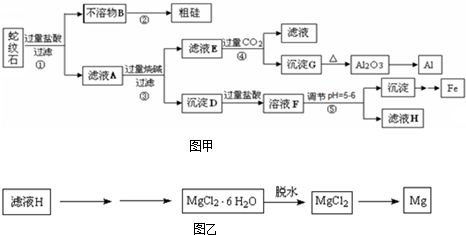
 ����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��
����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��