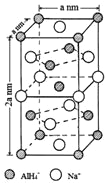
��Ŀ����
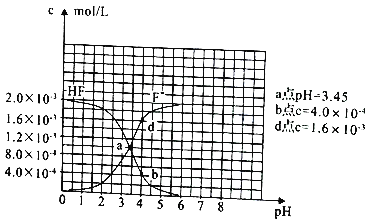
����Ŀ��H2RO3��һ�ֶ�Ԫ�ᣬ��������1L1mol��L-1Na2RO3��Һ����RO2���壬��Һ��pH��RO2��������ʵ����ı仯��ͼ��ʾ������˵����ȷ����
A. a����Һ��2c(Na+)=3c(RO32-)
B. ��b����Һ�м�ˮ��ʹ��Һ��pH��6.2���ߵ�7.4
C. �����£�NaHRO3��Һ��c(HRO3-)>c(RO32-)>c(H2RO3)
D. ������RO2����Һ������ʱ��c(Na+)=c(RO32-)+c(HRO3-)
���𰸡�C
��������a����Һ�к���H2RO31mol��H2RO3+H2O+RO2=2NaHRO3 ����a��ͨ��1/3 mol RO2����Һ������������2mol��RO32-Ϊ2/3 mol,��ʱa����Һ�ʼ��ԣ�˵��RO32-���ӷ�����ˮ�⣬��2c(Na+)>3c(RO32-)��A������b�㵼����Һ���Ե�������HRO3-�ĵ��룬�����Һ��ˮ�����ɵ�������Ũ����С��pH��ֻ�����ӽ�7�����ᳬ��7��B��������ͨ��5/6 molRO2ʱ����Һ������5/3mol NaHRO3 ��ʣ��Na2RO3Ϊ1/6 mol����ʱ��Һ��pH=6.2�����ԣ�˵��HRO3-�Ե���Ϊ�����ʳ����£�NaHRO3��Һ�У�c(HRO3-)>c(RO32-)>c(H2RO3)��C��ȷ��������RO2����Һ������ʱ�����ݵ���غ㣺c(H+)+c(Na+)=2c(RO32-)+c(HRO3-)+c(OH-)������c(H+)=c(OH-)������c(Na+)=2c(RO32-)+c(HRO3-)��D��������ȷѡ��C��

����Ŀ����������Ҫ����ԭ�ϣ��ڹ�����ռ��Ҫ��λ����ҵ�ϳɰ��ķ�ӦΪ: N2(g)+H2(g)=2NH3(g) ��H<0
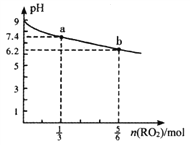
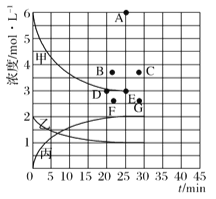
��1����ͼ��ʾ�ϳ�NH3��Ӧ��ij��ʱ��t0��t6�з�Ӧ�����뷴Ӧ���̵�����ͼ��t1��t3��t4 ʱ�̷ֱ�ı�ijһ����������������е��ﻯѧƽ���ʱ����У�NH3�����������С��һ��ʱ����___________(��д�������)

A.t1��t1 B.t2��t3 C.t3��t4 D.t5��t6
t4ʱ�ı��������________________��
���������о�:��773Kʱ���ֱ�2molN2��6molH2����һ���̶��ݻ�Ϊ1L���ܱ������У����ŷ�Ӧ�Ľ��У�����������n(H2)��n(NH3)�뷴Ӧʱ��t�Ĺ�ϵ���±���
t/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
n(H2)/mol | 6.00 | 4.50 | 3.60 | 3.30 | 3.03 | 3.00 | 3.00 |
n(NH3)/mol | 0 | 1.00 | 1.60 | 1.80 | 1.98 | 2.00 | 2.00 |
��2�����¶��£��˷�Ӧ��ƽ�ⳣ��K=____________��
��3�����¶��£�����ͬ�ݻ�����һ������Ͷ���N2��H2��NH3��Ũ�ȷֱ�Ϊ3mo/L��3mol/L��3mo/L�����ʱV��_____V�� (��">"��<������=��)��
��4�����ϱ��е�ʵ�����ݼ���õ���Ũ��һʱ�����Ĺ�ϵ������ͼ�е����߱�ʾ����ʾc(N2)-t��������______���ڴ��¶��£�����ʼ����4molN2��12 molH2����Ӧ�մﵽƽ��ʱ����ʾc(H2)-t����������Ӧ�ĵ�Ϊ_________��
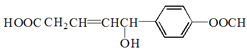
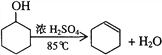
����Ŀ��ij��ѧС���������������������װ�ã��û������Ʊ�����ϩ��
��֪�� 
�ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
������ | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 0.81 | ��103 | 83 | ������ˮ |
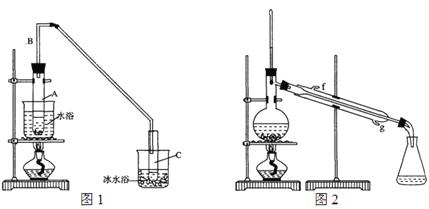
��1���Ʊ���Ʒ��������ͼ1��ʾװ�ã��û������Ʊ�����ϩ��
��12.5 mL�����������Թ�A�У��ټ���1 mLŨ���ᣬҡ�ȷ������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������_____________������B��������_______________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����________________________��
��2���Ʊ���Ʒ��
������ϩ��Ʒ�к��л������������л��������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��______�㣨��ϡ����¡�������Һ����________ϴ�ӣ�����ĸ����
A. KMnO4��Һ B. ϡH2SO4 C. Na2CO3��Һ
���ٽ�����ϩ��ͼ2װ��������ȴˮ��____�ڣ�����ĸ�����롣����ʱ������ʯ�ң�Ŀ����______________________________________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��______���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����______������ĸ����
a. ����ʱ��70����ʼ�ռ���Ʒ
b. ������ʵ����������
c. �Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������________������ĸ����
a. �����Ը��������Һ
b. �ý�����
c. �ⶨ�е�