��Ŀ����
X��Y��ZΪ���ֶ�����Ԫ�أ����������ڱ��е�λ�ù�ϵ��ͼ��ʾ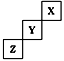 ���ݴ˻ش��������⣺
���ݴ˻ش��������⣺
��1��XԪ�ص�����Ϊ �����γɵĵ���Ϊ ���÷���ʽ��ʾ���� He ������Ԫ�ص�ij��ԭ���ں�������������������ȣ����ԭ�ӵ�Ħ�������� �����������Ƿ��ص�ͼƬԤ�⣬�����������������������ֵ�![]() ������ԭ����
������ԭ����![]() �Ĺ�ϵ�� ��
�Ĺ�ϵ�� ��
��2��YԪ���γɵ��ʵĵ���ʽ�� ��Y���ʵ������� ���ǿ�ڡ����ߡ����ڡ���O2�������ԣ�������Ӧ�Ļ�ѧ����ʽ˵�� ��
��3��Ԫ��Z����Ȼ���еĴ�����̬Ϊ ������ţ���ֻ�л���̬����ֻ������̬���ۼ�������̬�����л���̬����ZԪ�ص�ijЩ������ȼ�պ��������صĴ�����Ⱦ������Ҫԭ���� ��
��4��YԪ�ء�ZԪ����H2����ʱ�������仯�� ����������ȡ����������ȡ�����ǰ�߷��ȣ��������ȡ����ߡ�ǰ�����ȣ����߷��ȡ����������仯�Ĵ�С��ϵΪǰ�� ������������������ߡ���������
��1������He ��4g/mol ��ͬλ�أ���2�� ![]() ��ǿ�ڣ�2F2 + 2H2O = 4HF + O2����3���ۣ���S��ijЩ������ȼ�պ�����SO2�����γ����꣬Σ�������� ��4�������ȣ�����
��ǿ�ڣ�2F2 + 2H2O = 4HF + O2����3���ۣ���S��ijЩ������ȼ�պ�����SO2�����γ����꣬Σ�������� ��4�������ȣ�����
����:
������ͼ��ʾλ�õĶ�����Ԫ�أ����жϳ�X��Y��Z�ֱ�Ϊ He ��F��S��Heֻ���γɵ�ԭ�ӷ��ӣ���������ͬ����������ͬ������ԭ�ӻ�Ϊͬλ�أ���ɽ�ڸ������ڵ���S��SԪ��ȼ�պ�������Ⱦ������SO2��Ԫ�صķǽ�����Խǿ�����ɵ��⻯��Խ�ȶ����ų�������Խ�ࡣ






