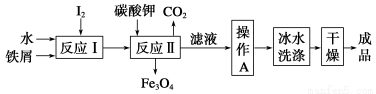
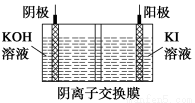
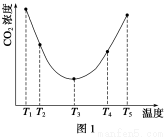
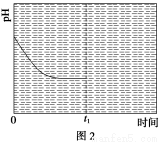
ΧβΡΩΡΎ»ί
“―÷Σ2RCH2CHO 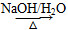
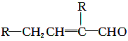
Υ°―νΥαθΞEΈΣΉœΆβœΏΈϋ ’ΦΝΘ§Ω…”Ο”Ύ≈δ÷ΤΖά…ΙΥΣΓΘEΒΡ“Μ÷÷Κœ≥…¬ΖœΏ»γœ¬ΘΚ
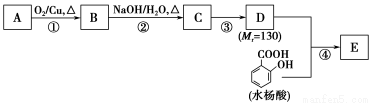
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)“Μ‘Σ¥ΦA÷–―θΒΡ÷ ΝΩΖ÷ ΐ‘ΦΈΣ21.6%Θ§‘ρAΒΡΖ÷Ή” ΫΈΣ________ΘΜΫαΙΙΖ÷Έωœ‘ ΨA÷Μ”–“ΜΗωΦΉΜυΘ§AΒΡΟϊ≥ΤΈΣ________ΓΘ
(2)BΡή”κ–¬÷ΤΒΡCu(OH)2ΖΔ…ζΖ¥”ΠΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________________________ΓΘ
(3)C”–________÷÷ΫαΙΙΘΜ»τ“Μ¥Έ»Γ―υΘ§Φλ―ιC÷–ΥυΚ§ΙΌΡήΆ≈Θ§Α¥ Ι”ΟΒΡœ»ΚσΥ≥–ρ–¥≥ωΥυ”Ο ‘ΦΝΘΚ______________________________________________ΓΘ
(4)ΒΎΔέ≤ΫΒΡΖ¥”Πάύ–ΆΈΣ________ΘΜDΥυΚ§ΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣ________ΓΘ
(5)–¥≥ωΆ§ ±ΖϊΚœœ¬Ν–ΧθΦΰΒΡΥ°―νΥαΥυ”–Ά§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΘΚ_____________________________ΓΘ
aΘ°Ζ÷Ή”÷–”–6ΗωΧΦ‘≠Ή”‘Ύ“ΜΧθ÷±œΏ…œΘΜ
bΘ°Ζ÷Ή”÷–ΥυΚ§ΙΌΡήΆ≈Αϋά®Υ°―νΥαΨΏ”–ΒΡΙΌΡήΆ≈ΓΘ
(6)ΒΎΔή≤ΫΒΡΖ¥”ΠΧθΦΰΈΣ______________ΘΜ–¥≥ωEΒΡΫαΙΙΦρ ΫΘΚ________________ΓΘ
(1)C4H10O
1?ΕΓ¥Φ(Μρ’ΐΕΓ¥Φ)
(2)CH3CH2CH2CHOΘΪ2Cu(OH)2ΘΪNaOH CH3CH2CH2COONaΘΪCu2OΓΐΘΪ3H2O
CH3CH2CH2COONaΘΪCu2OΓΐΘΪ3H2O
(3)2ΓΓ“χΑ±»ή“ΚΓΔœΓ―ΈΥαΓΔδεΥ°(ΜρΤδΥϊΚœάμ¥πΑΗ)
(4)ΜΙ‘≠Ζ¥”Π(ΜρΦ”≥…Ζ¥”Π)ΓΓτ«Μυ
(5) 

(6)≈®H2SO4Θ§Φ”»»

ΓΨΫβΈωΓΩΗυΨίΧαΙ©–≈œΔΓΔΉΣΜ·ΧθΦΰ»ΖΕ®Ζ¥”ΠΈοΦΑΖ¥”Πάύ–ΆΓΘ
(1)AΈΣ“Μ‘Σ¥ΦΘ§Τδ÷–―θΒΡ÷ ΝΩΖ÷ ΐ‘ΦΈΣ21.6%Θ§Υυ“‘œύΕ‘Ζ÷Ή”÷ ΝΩMrΘΫ16Γ¬21.6%Γ÷74ΓΘ…ηΖ÷Ή” ΫΈΣCxHyOΘ§‘ρ12xΘΪyΘΪ16ΘΫ74,12xΘΪyΘΫ58ΓΘΒ±xΘΫ4 ±Θ§yΘΫ10Θ§Ζ÷Ή” ΫΈΣC4H10OΓΘΒ±xΘΫ3ΚΆxΘΫ5 ±Ψυ≤Μ≥…ΝΔΘ§Υυ“‘AΒΡΖ÷Ή” ΫΈΣC4H10OΓΘA÷–÷Μ”–“ΜΗωΦΉΜυΘ§Υυ“‘AΈΣ
CH3CH2CH2CH2OHΘ§ΟϋΟϊΈΣ1?ΕΓ¥Φ(Μρ’ΐΕΓ¥Φ)ΓΘ
(2)”…ΔΌΧθΦΰΩ…÷ΣBΈΣCH3CH2CH2CHOΓΘB”κ–¬÷ΤΒΡCu(OH)2ΒΡΖ¥”ΠΈΣCH3CH2CH2CHOΘΪ2Cu(OH)2ΘΪNaOH CH3CH2CH2COONaΘΪCu2OΓΐΘΪ3H2OΓΘ
CH3CH2CH2COONaΘΪCu2OΓΐΘΪ3H2OΓΘ
(3)”…ΧβΗ…ΧαΙ©ΒΡ–≈œΔΩ…÷ΣΔΎΒΡΖ¥”ΠΙΐ≥ΧΈΣ
2CH3CH2CH2CHO

Υυ“‘C”–2÷÷ΫαΙΙΓΘΓΣCHOΚΆ Ι≤Ά§¥φ‘Ύ ±“Σœ»Φλ―ιΓΣCHOΘ§»ΜΚσΦλ―ι
Ι≤Ά§¥φ‘Ύ ±“Σœ»Φλ―ιΓΣCHOΘ§»ΜΚσΦλ―ι Θ§Υυ”Ο ‘ΦΝΈΣ“χΑ±»ή“ΚΓΔœΓ―ΈΥαΚΆδεΥ°ΓΘ
Θ§Υυ”Ο ‘ΦΝΈΣ“χΑ±»ή“ΚΓΔœΓ―ΈΥαΚΆδεΥ°ΓΘ
(4)CΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ126Θ§DΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ130Θ§Υυ“‘C®DΓζD «”κH2ΖΔ…ζΦ”≥…Ζ¥”ΠΘ§“≤≥ΤΈΣΜΙ‘≠Ζ¥”ΠΓΘ”…”ΎœύΕ‘Ζ÷Ή”÷ ΝΩœύ≤ν4Θ§Υυ“‘ ΚΆΓΣCHOΕΦ”κH2Φ”≥…Θ§Υυ“‘D÷–ΙΌΡήΆ≈Οϊ≥ΤΈΣτ«ΜυΓΘ
ΚΆΓΣCHOΕΦ”κH2Φ”≥…Θ§Υυ“‘D÷–ΙΌΡήΆ≈Οϊ≥ΤΈΣτ«ΜυΓΘ
(5)  ΒΡ≤Μ±ΞΚΆΕ»ΈΣ5Θ§ΥϋΒΡΆ§Ζ÷“λΙΙΧεΖϊΚœ6ΗωΧΦ‘≠Ή”‘Ύ“ΜΧθ÷±œΏ…œΘ§Ά§ ±ΜΙΚ§”–ΓΣCOOHΚΆΓΣOHΘ§Ω…“‘‘ΎΧΦΝ¥…œ“ΐ»κ»ΐΦϋΜρΥΪΦϋΓΘΓΣCOOHΈΣ“ΜΗω≤Μ±ΞΚΆΕ»Θ§ΜΙΩ…“ΐ»κ2Ηω»ΐΦϋΜρ4ΗωΥΪΦϋΓΘ”…”Ύ“Σ«σ6ΗωΧΦ‘≠Ή”‘Ύ“ΜΧθ÷±œΏ…œΘ§Υυ“‘“Σ«σ¥φ‘ΎΓΣCΓ‘CΓΣCΓ‘CΓΣ’β―υΒΡΫαΙΙΘ§’β―υΜΙ”ύœ¬ΓΣCOOHΓΔΓΣOHΓΔΓΣCH3ΚΆCHΜρΓΣCOOHΓΔΓΣOHΚΆ2ΗωΓΣCH2ΓΣΘ§Υυ“‘Ω…ΡήΒΡΫαΙΙ”–ΘΚ
ΒΡ≤Μ±ΞΚΆΕ»ΈΣ5Θ§ΥϋΒΡΆ§Ζ÷“λΙΙΧεΖϊΚœ6ΗωΧΦ‘≠Ή”‘Ύ“ΜΧθ÷±œΏ…œΘ§Ά§ ±ΜΙΚ§”–ΓΣCOOHΚΆΓΣOHΘ§Ω…“‘‘ΎΧΦΝ¥…œ“ΐ»κ»ΐΦϋΜρΥΪΦϋΓΘΓΣCOOHΈΣ“ΜΗω≤Μ±ΞΚΆΕ»Θ§ΜΙΩ…“ΐ»κ2Ηω»ΐΦϋΜρ4ΗωΥΪΦϋΓΘ”…”Ύ“Σ«σ6ΗωΧΦ‘≠Ή”‘Ύ“ΜΧθ÷±œΏ…œΘ§Υυ“‘“Σ«σ¥φ‘ΎΓΣCΓ‘CΓΣCΓ‘CΓΣ’β―υΒΡΫαΙΙΘ§’β―υΜΙ”ύœ¬ΓΣCOOHΓΔΓΣOHΓΔΓΣCH3ΚΆCHΜρΓΣCOOHΓΔΓΣOHΚΆ2ΗωΓΣCH2ΓΣΘ§Υυ“‘Ω…ΡήΒΡΫαΙΙ”–ΘΚ
 ΓΘ
ΓΘ
(6)ΒΎΔή≤ΫΈΣθΞΜ·Ζ¥”ΠΘ§Υυ“‘ΧθΦΰ «≈®H2SO4ΓΔΦ”»»Θ§EΒΡΫαΙΙΦρ ΫΈΣ
 ΓΘ
ΓΘ

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ