��Ŀ����
13��Ԫ��Xλ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2��Ԫ��Y��̬ԭ�ӵ�3p�������4�����ӣ�Ԫ��Z��̬ԭ�ӵ�2p�������3��δ�ɶԵ��ӣ�����˵����ȷ���ǣ�������| A�� | X���Ȼ����백ˮ��Ӧ���γ������[X��NH3��4]Cl2��1mol��������к���12mol �Ҽ� | |
| B�� | Z����ۺ���������У��ǻ����ͷ��ǻ���������Ϊ1��1 | |
| C�� | Y����̬�⻯�������H-Y-H���DZ�Y����������������O-Y-O����С | |
| D�� | Z����̬�⻯���Y����̬�⻯���ȶ�������Ϊ�����Ӱ�� |
���� Ԫ��Xλ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ����ڲ������=2+8+18=28��������������Ϊ2�����Ը�ԭ����30�����ӣ�ΪZnԪ�أ�Ԫ��Y��̬ԭ�ӵ�3p�������4�����ӣ���������Ų�Ϊ1s22s22p63s23p4����Y��SԪ�أ�Ԫ��Z��̬ԭ�ӵ�2p�������3��δ�ɶԵ��ӣ���������Ų�Ϊ1s22s22p3����ZΪNԪ�أ��ݴ˽��
��� �⣺Ԫ��Xλ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ����ڲ������=2+8+18=28��������������Ϊ2�����Ը�ԭ����30�����ӣ�ΪZnԪ�أ�Ԫ��Y��̬ԭ�ӵ�3p�������4�����ӣ���������Ų�Ϊ1s22s22p63s23p4����Y��SԪ�أ�Ԫ��Z��̬ԭ�ӵ�2p�������3��δ�ɶԵ��ӣ���������Ų�Ϊ1s22s22p3����ZΪNԪ�أ�
A��Zn���Ȼ����백ˮ��Ӧ���γ������[Zn��NH3��4]Cl2��п�����백������֮���γ�4����λ��������������Nԭ����Hԭ��֮���γ�N-H�ۣ���1mol��������к���16mol �Ҽ�����A����
B��Z����ۺ�����ΪHNO3���������ǻ����ͷ��ǻ���������Ϊ1��2����B����
C��H2S��������ԭ�Ӽ۲���Ӷ���Ϊ2+$\frac{6-1��2}{2}$=4����2�Թµ��Ӷԣ�VSEPRģ��Ϊ�������壬���ӹ���ΪV�ͽṹ��SO2��������ԭ�Ӽ۲���Ӷ���Ϊ2+$\frac{6-2��2}{2}$=3����1�Թµ��Ӷԣ�VSEPRģ��Ϊƽ�������Σ����ӹ���ΪV�ͽṹ������������йµ��Ӷ�֮����ų������ڹµ��Ӷ�-�ɼ����ӶԵ��ų�������S����̬�⻯�������H-S-H���DZ�S����������������O-S-O����С����C��ȷ��
D��Z����̬�⻯���Y����̬�⻯���ȶ�������ΪNԪ�طǽ����Ա����ǿ�����Ӱ���������ʣ���D����
��ѡ��C��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų�����ѧ�������ӽṹ�����ʡ�����ȣ���Ҫѧ���߱���ʵ�Ļ�����C��ע������VSEPRģ�ͽ������⣬�Ѷ��еȣ�

| A�� | ����ʽ��ͬ���ṹʽ��ͬ�Ļ����ﻥ��ͬ���칹�� | |
| B�� | ���Ԫ����ͬ���ṹʽ��ͬ�����ʻ���ͬ���칹�� | |
| C�� | ��Ϊͬ���칹�������������ͬ | |
| D�� | ��Ϊͬ���칹��������������� |
| ���� | Cl2 | Br2 | I2 | H2 | HF | HCl HBr HI |
| ���� | 243 | 193 | 151 | 436 | 568 | 432 366 298 |
��1���������ʱ������е�������͵���A ����A��B��C��D��
A��H2 B��Cl2 C��Br2 D��I2
��2�������⻯���У����ȶ�����A ����A��B��C��D��
A��HF B��HCl C��HBr D��HI
��3��X2+H2=2HX��X����Cl��Br��I���ķ�Ӧ�Ƿ��ȷ�Ӧ������ȡ����ȡ���
��4����ͬ�����£�X2��X����Cl��Br��I���ֱ���������Ӧ�������ĵ����ʵ���������ʱ���ų������յ�����������Cl2����д����ʽ����
��5�������ϱ��е����ݣ�������ȷ�ش�����⣨4�����ܣ������ܡ�����ĸ�����Ԫ�صķǽ�����Խǿ��������Խ�ȶ����ų�������Խ�࣬���⼸��HX�У�HCl���ȶ����ų��������࣮
| A�� | H3O+ | B�� | NaOH | C�� | NH4Cl | D�� | H2SO4 |
| A�� | v��B��=0.075mol/��L•s�� | B�� | v��A��=0.030mol/��L•s�� | C�� | v��C��=0.040mol/��L•s�� | D�� | v��D��=0.060mol/��L•s�� |
| A�� | �����¶� | B�� | ʹ�ô��� | ||
| C�� | ����ϡ�����壬������ϵѹǿ | D�� | ����N2��H2����ʼ�� |
 ij�л���Ľṹ��ʽ��ͼ�������ܷ����ķ�Ӧ�����У���ȡ�� �ڼӳ� ����ȥ ��ˮ�� ������ ���к� ������ ��Ӿۣ�������
ij�л���Ľṹ��ʽ��ͼ�������ܷ����ķ�Ӧ�����У���ȡ�� �ڼӳ� ����ȥ ��ˮ�� ������ ���к� ������ ��Ӿۣ�������| A�� | �٢ڢۢޢ� | B�� | �ڢۢܢݢ� | C�� | �٢ڢۢݢޢ� | D�� | �ۢܢݢޢ� |
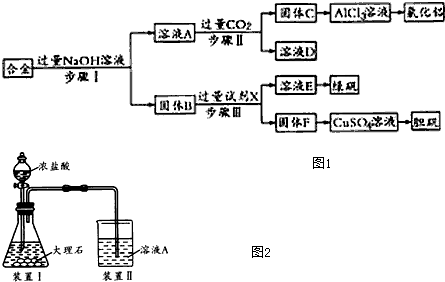
 �� 4���л���ֱ���һ����������H2��ַ�Ӧ��
�� 4���л���ֱ���һ����������H2��ַ�Ӧ�� ������ˮ��Ӧ���ɲ���Ľṹ��ʽΪ
������ˮ��Ӧ���ɲ���Ľṹ��ʽΪ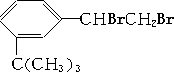 ��
��