��Ŀ����
��ͬԪ�ص�ԭ���ڷ������������ӵ�������С���õ縺������ʾ���縺��Խ����ԭ���������ӵ�����Խǿ�������γɵķ����г�Ϊ������ɵ�һ�����±���ijЩ������Ԫ�صĵ縺��ֵ

(1)ͨ�������縺�Ա仯���ɣ�ȷ��N��Mg��ӽ��ĵ縺�� ��Χ��___<Mg<___��____<N<______
(2)�Ʋ�縺����ԭ�Ӱ뾶�Ĺ�ϵ��____________���ϱ��ж�����Ԫ�ص縺�Եı仯�ص㣬������Ԫ�����ʵ�__________�仯���ɡ�
(3)ij�л�������ĽṹʽΪ ������C-N���У�����Ϊ���õ��Ӷ�ƫ����__________��дԭ�����ƣ�һ����
������C-N���У�����Ϊ���õ��Ӷ�ƫ����__________��дԭ�����ƣ�һ����
(4)������ɸ������ǣ����ɼ�����ԭ����ӦԪ�صĵ縺�Բ�ֵ����1.7ʱ��һ��Ϊ���Ӽ���С��1.7ʱ��һ��Ϊ���ۼ������ƶϣ�AlBr3�л�ѧ��������___________��
(1)0.9��1.5��2.5��3.5
(2)ԭ�Ӱ뾶Խ�縺��ԽС��������
(3)��
(4)���ۼ�
(2)ԭ�Ӱ뾶Խ�縺��ԽС��������
(3)��
(4)���ۼ�

��ϰ��ϵ�д�
�����Ŀ

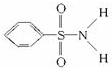 ������S-N�У�����Ϊ���õ��Ӷ�ƫ��
������S-N�У�����Ϊ���õ��Ӷ�ƫ��