��Ŀ����
����Ŀ�������Ѿ�ѧ���Ļ�ѧ֪ʶ���ش��������⡣
I.�������仯�������������������Ź㷺��Ӧ�ã�
(1)θ��ƽ(��Ҫ�ɷ�Ϊ��������)����������θ����࣬������������_______�ԣ�_______(��ܡ����ܡ�)������������Һ���档
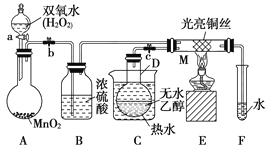
(2)��Cu��ϡ�����ϣ����߲��ܷ�Ӧ������H2O2����Һ�ܿ�����ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
(3)NaHSO4��һ����ʽ�Σ�д��NaHSO4��ˮ�еĵ��뷽��ʽ_________________________��
II.���ʵ�����ѧϰ��ѧ�Ļ�����
(1)14.4 g CO��CO2�Ļ�������ڱ�״������ռ�����Ϊ8.96 L������CO������Ϊ_____��
(2)����ŨH2SO4����������Ϊ98%���ܶ�Ϊ1.84g/cm3����Ũ��Ϊ________mol��L��1��
(3)19gij���۽������Ȼ���ACl2�к���0.4mo1Cl�����ӣ�����A�����ԭ��������_______��
(4)���ݷ�Ӧ14CuSO4��5FeS2��12H2O��7Cu2S��5FeSO4��12H2SO4����֪����2.5 mol FeS2�μӷ�Ӧʱ����������������ʵ���Ϊ_______mol��
���𰸡����� ���� Cu+H2SO4+H2O2=CuSO4+2H2O NaHSO4=Na++H++SO42- 5.6g 18.4 24 1.5
��������
I.��1��θ�����Ҫ�ɷ���HCl�������������������ԣ����к�θ�
��2��Cu��ϡ�����H2O2��ַ�Ӧ����������ͭ��ˮ��
��3��NaHSO4��һ����ʽ�Σ�Ϊǿ����ʣ���ȫ���룻
II.��1������������CO������Ϊxg��CO2������Ϊyg��������֪�����ж�Ԫһ�η����飬��һ�������CO��������
��2������c=![]() ����Ũ�����Ũ�ȣ�
����Ũ�����Ũ�ȣ�
��3������Cl�������ʵ��������ACl2�����ʵ���������n=![]() ����Ħ�����������õ�A�����ԭ��������
����Ħ�����������õ�A�����ԭ��������
��4����Ӧ��Cu��+2�۽��͵�+1�ۣ�S��-1�����ߵ�+6�ۣ����͵�-2�ۣ��ɴ˽��з����жϡ�
I.��1��θ�����Ҫ�ɷ���HCl�������������������ԣ��������Ƶļ��Թ�ǿ�����и�ʴ�ԣ�����к�θ��ʱ������������������Һ���棻
��2��Cu��ϡ�����H2O2��ַ�Ӧ����������ͭ��ˮ����Ӧ����ʽΪ��Cu+H2SO4+H2O2=CuSO4+2H2O��
��3��NaHSO4��һ����ʽ�Σ�Ϊǿ����ʣ���ȫ���룬���뷽��ʽΪ��NaHSO4=Na++H++SO42-��
II.��1������������CO������Ϊxg��CO2������Ϊyg��������֪�����ɵ�
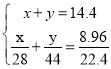 �����
�����![]() �����CO������Ϊ5.6g��
�����CO������Ϊ5.6g��
��2��c=![]() =
=![]() =18.4g/mol��
=18.4g/mol��
��3���Ȼ���ACl2�к���0.4mo1Cl������ôACl2�����ʵ���Ϊ0.2mo1��ACl2��Ħ������M=![]() =
=![]() =95g/mol�����A�����ԭ������Ϊ95-35.5��2=24��
=95g/mol�����A�����ԭ������Ϊ95-35.5��2=24��
��4����Ӧ��Cu��+2�۽��͵�+1�ۣ�S��-1�����ߵ�+6�ۣ����͵�-2�ۣ��ɵù�ϵʽ5FeS2~3S����˵���2.5mol FeS2�μӷ�Ӧʱ����������������ʵ���Ϊ2.5mol��![]() =1.5mol��
=1.5mol��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�