��Ŀ����
����ˮ�����׳ư�˾ƥ�֣�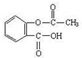 ������������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128�桫135�档ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣����Ʊ�ԭ��Ϊ��
������������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128�桫135�档ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣����Ʊ�ԭ��Ϊ��
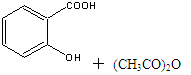

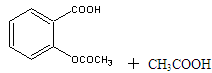
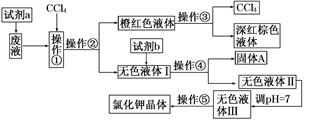
�Ʊ����������������£�
��Ҫ�Լ��Ͳ�Ʒ�������������±���
�ش��������⣺
��1���ϳɰ�˾ƥ��ʱ������ʵļ��ȷ����� ��
��2���ϳɰ�˾ƥ��ʱ������ʹ�ø������������ԭ���� ��
��3����ѹ�������ôֲ�ƷҪ��������ˮϴ�ӣ���Ŀ���� ��
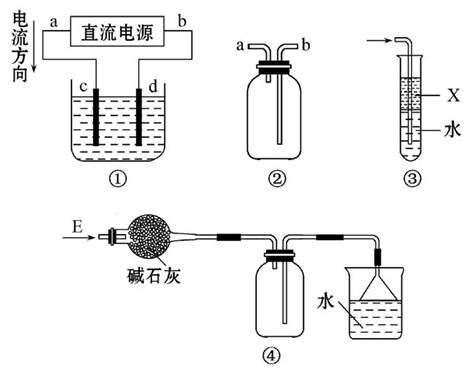
��4�����ؽᾧ�����ᴿ�ֲ�Ʒ�������£����Ȼ�����װ����ͼ��

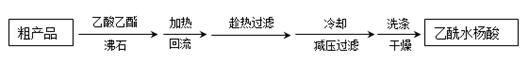
�ٷ�ʯ�������� ��
������ˮ������������ ���a����b������
��ʹ���¶ȼƵ�Ŀ���� ��
��5����ʵ����ԭ��������2.0gˮ���ᡢ5.0mL��������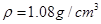 �������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ ���ٷ�����ȷ��0.1����
�������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ ���ٷ�����ȷ��0.1����
 ������������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128�桫135�档ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣����Ʊ�ԭ��Ϊ��
������������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128�桫135�档ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣����Ʊ�ԭ��Ϊ��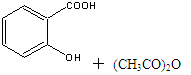

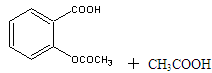
�Ʊ����������������£�

��Ҫ�Լ��Ͳ�Ʒ�������������±���
| ���� | ��Է������� | �۵��е㣨�棩 | ˮ |
| ˮ���� | 138 | 158���۵㣩 | �� |
| ������ | 102 | 139.4���е㣩 | ��Ӧ |
| ����ˮ���� | 180 | 135���۵㣩 | �� |
�ش��������⣺
��1���ϳɰ�˾ƥ��ʱ������ʵļ��ȷ����� ��
��2���ϳɰ�˾ƥ��ʱ������ʹ�ø������������ԭ���� ��
��3����ѹ�������ôֲ�ƷҪ��������ˮϴ�ӣ���Ŀ���� ��
��4�����ؽᾧ�����ᴿ�ֲ�Ʒ�������£����Ȼ�����װ����ͼ��


�ٷ�ʯ�������� ��
������ˮ������������ ���a����b������
��ʹ���¶ȼƵ�Ŀ���� ��
��5����ʵ����ԭ��������2.0gˮ���ᡢ5.0mL��������
 �������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ ���ٷ�����ȷ��0.1����
�������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ ���ٷ�����ȷ��0.1������1��ˮԡ����(2��)
��2����ֹ��������ˮ��Ӧ(2��)
��3����ȥ�����������ᡢ���ᣬ����������ˮ��������(2��)
��4���ٷ�ֹ����(2��)��a(2��)�۱��ڵ��ؼ����¶ȣ���ֹ����ˮ����ֽ�(2��)
��4��84.3%(��84.6%)(2��)
��2����ֹ��������ˮ��Ӧ(2��)
��3����ȥ�����������ᡢ���ᣬ����������ˮ��������(2��)
��4���ٷ�ֹ����(2��)��a(2��)�۱��ڵ��ؼ����¶ȣ���ֹ����ˮ����ֽ�(2��)
��4��84.3%(��84.6%)(2��)
�����������1�������¶���85�桫90�棬С��100�棬Ӧʹ��ˮԡ���ȣ�
��2���ϳɰ�˾ƥ��ʱ������ʹ�ø������������ԭ����������������ˮ�⣬���Դ��Ƿ�ֹ��������ˮ��Ӧ��
��3��ϴ�Ӿ��壬��ȥ��������ʣ�����ˮ��Ŀ���Ǽ�С��˾ƥ�ֵ��ܽ⣬���Դ��dz�ȥ�����������ᡢ���ᣬ����������ˮ�������ģ�
��4���ٷ�ʯ�������Ƿ�ֹ���У�
��Ϊʹ�������ڵIJ������õ������ȴ���ڽ�����ȴʱ��Ӧʹˮ�ķ���������ķ����෴��ʹˮ�������������ܣ���������ˮ������������a��
��ʹ���¶ȼƵ�Ŀ���DZ��ڵ��ؼ����¶ȣ���ֹ����ˮ����ֽ⣻
��4������ˮ���ᣩ=
 =0.0145mol��n����������=
=0.0145mol��n����������= =0.053mol��
=0.053mol������������������ˮ����0.0145mol������Ϊ
 ��100%=84.3%��
��100%=84.3%��
��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
�����Ŀ