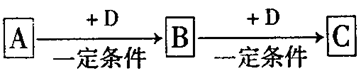��Ŀ����
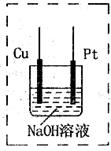
(9��)ij�ǽ�������A������ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺
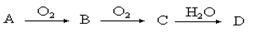
��1����A������Ϊ����ɫ���壬B���д̼�����ζ����ɫ���塣
��A��D�Ļ�ѧʽ�ֱ�Ϊ��A D
�ڹ�ҵ�����д����ŷŵ�B���屻��ˮ���պ��γ� ����Ⱦ������
��д��B��C��Ӧ�Ļ�ѧ����ʽ��
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��A�ĵ���ʽΪ ��C�Ļ�ѧʽΪ
��д��C��D��Ӧ�Ļ�ѧ����ʽ��

��1����A������Ϊ����ɫ���壬B���д̼�����ζ����ɫ���塣
��A��D�Ļ�ѧʽ�ֱ�Ϊ��A D
�ڹ�ҵ�����д����ŷŵ�B���屻��ˮ���պ��γ� ����Ⱦ������
��д��B��C��Ӧ�Ļ�ѧ����ʽ��
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��A�ĵ���ʽΪ ��C�Ļ�ѧʽΪ
��д��C��D��Ӧ�Ļ�ѧ����ʽ��
�� A S D H2SO4 �� ����
��2SO2+O2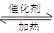 2SO3������ʽÿ��2�֣�����ÿ��1�ֹ�9�֣�
2SO3������ʽÿ��2�֣�����ÿ��1�ֹ�9�֣�
��2���� �� NO2 ��3 NO2+ H2O�� 2HNO3+ NO
�� NO2 ��3 NO2+ H2O�� 2HNO3+ NO
��2SO2+O2
 2SO3������ʽÿ��2�֣�����ÿ��1�ֹ�9�֣�
2SO3������ʽÿ��2�֣�����ÿ��1�ֹ�9�֣���2����
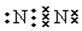 �� NO2 ��3 NO2+ H2O�� 2HNO3+ NO
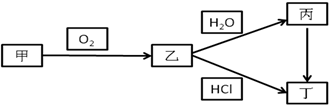
�� NO2 ��3 NO2+ H2O�� 2HNO3+ NO�����������1����A��һ�ֻ�ɫ���ʹ��壬��A��S���ʣ�����B�Ƕ�������C������������B��C�Ļ�ѧ����ʽΪ2SO2+O2
 2SO3��
2SO3����2������A�ڳ�����Ϊ���壬C�Ǻ���ɫ���壬��AӦ���ǵ�����C��NO2��B��NO��
��NO2��ˮ��Ӧ�Ļ�ѧ����ʽ��3 NO2+ H2O�� 2HNO3+ NO��
�����������Ǹ߿��еij������ͣ����ڻ���������Ŀ��顣�����������У�����֪ʶ�����ض�ѧ������֪ʶ�Ĺ��̸�ѵ��������������Ҫע����ǻ�ѧ�ƶ�����һ���ۺ��Խ�ǿ�����⣬��Ԫ�ؼ����������ʺ�������������������ѧ�����֪ʶ����������ѧ�Ƽ��ۺϡ��������ɿ���ѧ���Ի�ѧ֪ʶ������̶ȣ�����Ҫ��������ѧ�����ۺϷ���������˼ά���������ͼ��ķ�������ؼ�����Ѱ�ҡ�ͻ�ƿڡ�����ͻ�ƿڡ�����ץ���ء��֣�����������ɫ������״̬��������ζ�����ⷴӦ���������������Ʒ���������;�ȡ�

��ϰ��ϵ�д�
 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
�����Ŀ