��Ŀ����
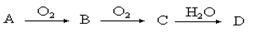
A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮��ת����ϵ����ͼ�����ֲ�������ȥ�����Իش�
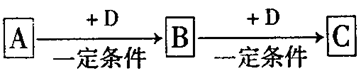
��1����D�Ǿ��������Եĵ��ʣ�����������Ľ���AΪ ________ ����Ԫ�ط��ţ���C�ĵ���ʽΪ__________
��2����A��ij���ʵ�ϡ��Һ��D�ǽ�����C��Һ�ڴ���ʱӦ��������D���������ǣ��ñ�Ҫ�����ֺ����ӷ���ʽ��ʾ��____________��D�ڳ�ʪ�Ŀ��������� ������ʴ��д����ʴʱԭ��������ĵ缫��Ӧʽ___________��

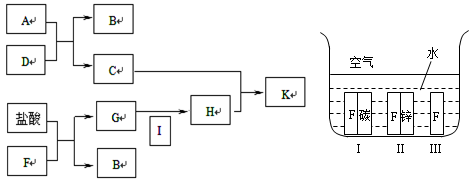
��3����A��B��CΪ��ͬһ�ֽ���Ԫ�ص������������Һ��A��C��Ӧ����B����д��Bת��ΪC�����п��ܵ����ӷ���ʽ_____________________��������B����һϵ�з�Ӧ���Եõ�����E����һ��������Mg��E �Ļ����Ͷ��500mLϡ�����У�����ȫ���ܽⲢ�������塣����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ����ͼ��ʾ�������������Mg������Ϊ________��NaOH��Һ�����ʵ���Ũ��Ϊ ________��������Һ�����ʵ���Ũ��Ϊ_______��
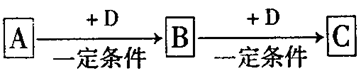
��1����D�Ǿ��������Եĵ��ʣ�����������Ľ���AΪ ________ ����Ԫ�ط��ţ���C�ĵ���ʽΪ__________
��2����A��ij���ʵ�ϡ��Һ��D�ǽ�����C��Һ�ڴ���ʱӦ��������D���������ǣ��ñ�Ҫ�����ֺ����ӷ���ʽ��ʾ��____________��D�ڳ�ʪ�Ŀ��������� ������ʴ��д����ʴʱԭ��������ĵ缫��Ӧʽ___________��

��3����A��B��CΪ��ͬһ�ֽ���Ԫ�ص������������Һ��A��C��Ӧ����B����д��Bת��ΪC�����п��ܵ����ӷ���ʽ_____________________��������B����һϵ�з�Ӧ���Եõ�����E����һ��������Mg��E �Ļ����Ͷ��500mLϡ�����У�����ȫ���ܽⲢ�������塣����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ����ͼ��ʾ�������������Mg������Ϊ________��NaOH��Һ�����ʵ���Ũ��Ϊ ________��������Һ�����ʵ���Ũ��Ϊ_______��
��1��Na 
(2)��������������ֹ������ ��2Fe3����Fe��3Fe2����O2��4e����2H2O��4OH��
��2Fe3����Fe��3Fe2����O2��4e����2H2O��4OH��
��3��Al(OH)3��3H����Al3����3H2O��Al(OH)3��OH����AlO2����2H2O
3.6g ��5.0mol/L ��1.0mol/L

(2)��������������ֹ������
 ��2Fe3����Fe��3Fe2����O2��4e����2H2O��4OH��
��2Fe3����Fe��3Fe2����O2��4e����2H2O��4OH����3��Al(OH)3��3H����Al3����3H2O��Al(OH)3��OH����AlO2����2H2O
3.6g ��5.0mol/L ��1.0mol/L
�����������1����D�Ǿ��������Եĵ��ʣ���������ʵ�ת����ϵ�Լ�ת���ص��֪����������Ľ���AΪNa����B�������ƣ�C���ǹ������ơ����������Ǻ������Ӽ��ͷǼ��Լ������ӻ���������ʽΪ
 ��
����2����A��ij���ʵ�ϡ��Һ��D�ǽ��������Ը���ת���Ĺ�ϵ�Լ�ת���ص���ж�DӦ���DZ�۽�������B�Ǻ��������ӵ��Σ�C�Ǻ����������ӵ��Ρ��������������ױ��������������ӣ�����C��Һ�ڴ���ʱӦ������������Ŀ����Ϊ�˷�ֹ�������ӱ���������Ӧ�ķ���ʽ��2Fe3����Fe��3Fe2���������ڷ���������ʴʱ�����Ǹ�����ʧȥ���ӣ������������õ����ӣ����������缫��Ӧʽ��O2��4e����2H2O��4OH����
��3����A��B��CΪ��ͬһ�ֽ���Ԫ�ص������������Һ��A��C��Ӧ����B����˵�����ֻ����ﶼ������Ԫ�أ�����B���������������D���ᣬ��A����ƫ�����Σ�C�����Ρ����D�Ǽ��A�������Σ�C��ƫ�����Σ�����Bת��ΪC�����п��ܵ����ӷ���ʽAl(OH)3��3H����Al3����3H2O��Al(OH)3��OH����AlO2����2H2O��E�ǵ�����������ͼ���֪����������������Һ��û���������ɳ�������˵��ϡ�����ǹ����ģ������йصķ���ʽ��H����OH��=H2O��Al3����3OH��=Al(OH)3����Mg2����2OH��=Mg(OH)2����Al(OH)3��OH��=AlO2����2H2O������ͼ���֪��������������Ӧ������������Һ��240ml��200ml��40ml�����������������ʵ�����0.35mol��0.15mol��0.20mol��������þ��0.15mol�����Ի����þ������0.15mol��24g/mol3.6g������������Һ�����ʵ���Ũ����0.2mol��0.04L��5.0mol/L�������������ﵽ���ֵʱ��Һ�о�ֻ�������ƣ������������Ƶ����ʵ�����0.2L��5mol/L��1mol����˸���ԭ���غ��֪�������Ƶ����ʵ�����0.5mol���������Ƶ�Ũ����0.5mol��0.5L��1.0mol/L��
�����������Ǹ߿��еij������ͺͿ��㣬�����е��Ѷȵ����⡣�����ۺ���ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ�������������������Ӧ���������ڽ�������ͼ����ж�ʱӦ��ע����ǻ�ѧ�ƶ�����һ���ۺ��Խ�ǿ�����⣬��Ԫ�ؼ����������ʺ�������������������ѧ�����֪ʶ����������ѧ�Ƽ��ۺϡ��������ɿ���ѧ���Ի�ѧ֪ʶ������̶ȣ�����Ҫ��������ѧ�����ۺϷ���������˼ά���������ͼ��ķ�������ؼ�����Ѱ�ҡ�ͻ�ƿڡ�����ͻ�ƿڡ�����ץ���ء��֣�����������ɫ������״̬��������ζ�����ⷴӦ���������������Ʒ���������;�ȡ�����ļ���ؼ�����ȷ��Ӧԭ����Ȼ�����ݷ���ʽ��ͼ��������ü��ɡ�

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ