��Ŀ����
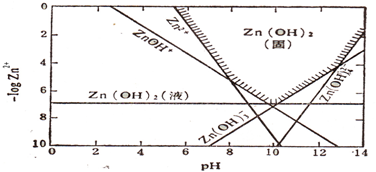
��2010?������ģ�⣩��ˮ��Һ�и۽��������磺Fe3+��Al3+�Ȼᷢ��ǿ��ˮ�⣬ͬ�����۽���������Cu2+��Pb2+��Zn2+��������һ����pH��Χ��Ҳ����ˮ�⣮������������ij���ֱ������ҺpH�Ŀ��ƣ�������һ�������Գ�ȥ��ҵ��ˮ�е�ijЩ�������ӣ���ͼ��Zn��OH��2����ij�¶�ʱ�ij�����������ͼ
��ش��������⣺
��1����һ��Ũ�ȵ�Zn2+����Һ�м���������NaOH���壬����˵����ȷ����
A��һ���������� B���Ȳ����������������ʧ
C��һ������������ D�����ܲ���������
��2����pH=6��c��Zn2+��=0.01mol?L-1��Zn2+��Ҫ������ʽΪ
��3����c��Zn2+��=1��10-5mol?L-1����Һ�м������NaOH��pH=10��pH=13��д���йط�Ӧ�����ӷ���ʽ����
��4����֪���¶��µ�Ksp[Zn��OH��2]=1��10-17�����㣺����Һ�е�c��Zn2+��=1��10-5mol?L-1������Һ�м���NaOH���壬����������pH�������ո�ͼ�ж��Ƿ���IJ���������ԭ�������ʲô��

��ش��������⣺
��1����һ��Ũ�ȵ�Zn2+����Һ�м���������NaOH���壬����˵����ȷ����
D
D
A��һ���������� B���Ȳ����������������ʧ
C��һ������������ D�����ܲ���������
��2����pH=6��c��Zn2+��=0.01mol?L-1��Zn2+��Ҫ������ʽΪ
ZnOH+
ZnOH+
��Zn��OH��2Һ��
Zn��OH��2Һ��
����3����c��Zn2+��=1��10-5mol?L-1����Һ�м������NaOH��pH=10��pH=13��д���йط�Ӧ�����ӷ���ʽ����
Zn2++2OH-�TZn��OH��2��
Zn2++2OH-�TZn��OH��2��
����Zn2++4OH-�TZn��OH��2-4
Zn2++4OH-�TZn��OH��2-4
��4����֪���¶��µ�Ksp[Zn��OH��2]=1��10-17�����㣺����Һ�е�c��Zn2+��=1��10-5mol?L-1������Һ�м���NaOH���壬����������pH�������ո�ͼ�ж��Ƿ���IJ���������ԭ�������ʲô��
��������1������ͼ�������������NaOH������룬��pH�仯��Һ�п�ʼ��������pH����pH��6ʱ����Һ�п�ʼ���ֳ�������ҺpHΪ14ʱ�����������ܽ⣬�����ֲ�������г�����Ҳ����������
��2��PH=6��c��Zn2+��=0.01mol?L-1�����ú��������������ֵ�ҳ���Ӧ�����ӣ�����ͼ�����ͼ��Χ��п�Ĵ�����ʽΪ��ZnOH+��Zn��OH��2Һ�壻
��3������ͼ�����c��Zn2+��=1��10-5mol?L-1��PH=10ʱ��������������п�����Ĺ��̣�PH=13��������п�ܽ�Ĺ��̣�������������ֵ�������������ֵ���ж����ɵ����ʣ���д���ӷ���ʽ��
��4�������ܶȻ�������������������Ũ�ȣ�����PH����ͼ������ͺ���ķ�Χ�жϴ�����ʽ������ͼ����������Ĵ������
��2��PH=6��c��Zn2+��=0.01mol?L-1�����ú��������������ֵ�ҳ���Ӧ�����ӣ�����ͼ�����ͼ��Χ��п�Ĵ�����ʽΪ��ZnOH+��Zn��OH��2Һ�壻
��3������ͼ�����c��Zn2+��=1��10-5mol?L-1��PH=10ʱ��������������п�����Ĺ��̣�PH=13��������п�ܽ�Ĺ��̣�������������ֵ�������������ֵ���ж����ɵ����ʣ���д���ӷ���ʽ��
��4�������ܶȻ�������������������Ũ�ȣ�����PH����ͼ������ͺ���ķ�Χ�жϴ�����ʽ������ͼ����������Ĵ������
����⣺��1������ͼ�������������NaOH������룬��pH�仯��Һ�п�ʼ��������pH����pH��6ʱ����Һ�п�ʼ���ֳ�����pH��10ʱ������ʼ�ܽ⣬��ҺpHΪ14ʱ�����������ܽ⣬�����ֲ�������г�����Ҳ�����������ʴ�Ϊ��D��
��2����pH=6��c��Zn2+��=0.01mol?L-1��-lgc��Zn2+��=2������ͼ����������ͺ���������Ӧ�������ж�ΪZn2+��Zn��OH��2Һ�壬�ʴ�Ϊ��ZnOH+��Zn��OH��2Һ�壻
��3������c��Zn2+��=1��10-5mol?L-1����Һ�м������NaOH��pH=10��-lgc��Zn2+��=5������ͼ����������ͺ���������Ӧ�������ж�ΪZn��OH��2��
�ʴ�Ϊ��Zn2++2OH-�TZn��OH��2����
����c��Zn2+��=1��10-5mol?L-1����Һ�м������NaOH��pH=13��-lgc��Zn2+��=5������ͼ����������ͺ���������Ӧ�������ж�ΪZn��OH��42-����Ӧ�����ӷ���ʽΪZn2++4OH-�TZn��OH��42-��ֲ�д��Zn2++2OH-�TZn��OH��2����Zn��OH��2+2OH-�TZn��OH��42-��
�ʴ�Ϊ��Zn2++2OH-�TZn��OH��2����Zn2++4OH-�TZn��OH��42-��ֲ�д��Zn2++2OH-�TZn��OH��2�� Zn��OH��2+2OH-�TZn��OH��42-��
��4��������Zn��OH��2��Һ�����ܽ�ƽ�⣺Zn��OH��2��s��?Zn2+��aq��+2OH-��aq����Ksp��Zn��OH��2��=c��Zn2+��c2��OH-��=1��10-17����Һ�е�c��Zn2+��=1��10-5mol?L-1�����c��OH-��=10-6mol/L��c��H+��=1��10-8mol/L��pH=8��-lgc��Zn2+��=5����ʱ�������ɣ�п�Ĵ�����ʽΪZnOH+��
������������Zn2+����Һ�н��OH--�γ�ZnOH+�ȣ�ʹZn2+��Ũ�ȼ��٣����γɳ�����
��2����pH=6��c��Zn2+��=0.01mol?L-1��-lgc��Zn2+��=2������ͼ����������ͺ���������Ӧ�������ж�ΪZn2+��Zn��OH��2Һ�壬�ʴ�Ϊ��ZnOH+��Zn��OH��2Һ�壻
��3������c��Zn2+��=1��10-5mol?L-1����Һ�м������NaOH��pH=10��-lgc��Zn2+��=5������ͼ����������ͺ���������Ӧ�������ж�ΪZn��OH��2��
�ʴ�Ϊ��Zn2++2OH-�TZn��OH��2����
����c��Zn2+��=1��10-5mol?L-1����Һ�м������NaOH��pH=13��-lgc��Zn2+��=5������ͼ����������ͺ���������Ӧ�������ж�ΪZn��OH��42-����Ӧ�����ӷ���ʽΪZn2++4OH-�TZn��OH��42-��ֲ�д��Zn2++2OH-�TZn��OH��2����Zn��OH��2+2OH-�TZn��OH��42-��
�ʴ�Ϊ��Zn2++2OH-�TZn��OH��2����Zn2++4OH-�TZn��OH��42-��ֲ�д��Zn2++2OH-�TZn��OH��2�� Zn��OH��2+2OH-�TZn��OH��42-��
��4��������Zn��OH��2��Һ�����ܽ�ƽ�⣺Zn��OH��2��s��?Zn2+��aq��+2OH-��aq����Ksp��Zn��OH��2��=c��Zn2+��c2��OH-��=1��10-17����Һ�е�c��Zn2+��=1��10-5mol?L-1�����c��OH-��=10-6mol/L��c��H+��=1��10-8mol/L��pH=8��-lgc��Zn2+��=5����ʱ�������ɣ�п�Ĵ�����ʽΪZnOH+��
������������Zn2+����Һ�н��OH--�γ�ZnOH+�ȣ�ʹZn2+��Ũ�ȼ��٣����γɳ�����
���������⿼���˳����ܽ�ƽ��Ӧ�ã����Ӵ��ڵ��жϷ��������ͼ��ķ���п�����ڲ�ͬPH��Χ�ڵĴ�����ʽ������ƽ����Ksp��Ӧ�ã�����ת������������Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 ��R1��R2��R3����������
��R1��R2��R3����������

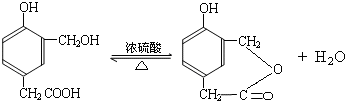
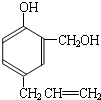
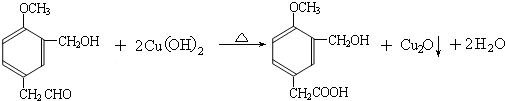
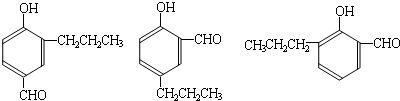
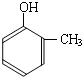 ���ĺϳ�
���ĺϳ�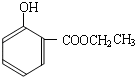 ·�ߣ��úϳ�·������ͼ��ʾ����ע����Ӧ��������
·�ߣ��úϳ�·������ͼ��ʾ����ע����Ӧ��������
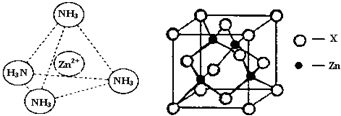
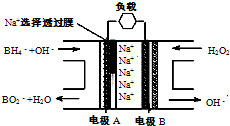 ��2010?������ģ�⣩ֱ��NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ�����������ϲ���Pt/C���������ϲ���MnO2�����йظõ�ص�˵����ȷ����
��2010?������ģ�⣩ֱ��NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ�����������ϲ���Pt/C���������ϲ���MnO2�����йظõ�ص�˵����ȷ����