��Ŀ����
��2010?������ģ�⣩��1��CH3+��CH3-��CH3-������Ҫ���л���Ӧ�м��壬�й����ǵ�˵����ȷ����
A�����Ǿ��ɼ���ȥ��һ����ԭ������
B�����ǻ�Ϊ�ȵ����壬̼ԭ�Ӿ���ȡsp2�ӻ�
C��CH3-��NH3��H3O+��Ϊ�ȵ����壬���ι��;�Ϊ������
D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ�����
E������CH3-��һ��CH3+��CH3-��Ͼ��ɵõ�CH3CH3
��2��п��һ����Ҫ�Ľ�����п���仯�������Ź㷺��Ӧ�ã�
��ָ��п�����ڱ��е�λ�ã�
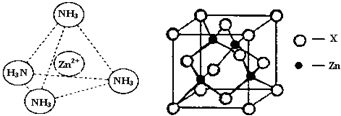
����������п[CH2OH��CHOH��4COO]2Zn��Ŀǰ�г������еIJ�п����д��Zn2+��̬�����Ų�ʽ
��Zn2+����NH3�������[Zn��NH3��4]2+�����NH3��������
����ͼ��ʾп��ij�ǽ���Ԫ��X�γɵĻ����ᄃ��������Zn��Xͨ�����ۼ���ϣ��û�����Ļ�ѧʽΪ
CDE
CDE
��A�����Ǿ��ɼ���ȥ��һ����ԭ������
B�����ǻ�Ϊ�ȵ����壬̼ԭ�Ӿ���ȡsp2�ӻ�
C��CH3-��NH3��H3O+��Ϊ�ȵ����壬���ι��;�Ϊ������
D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ�����
E������CH3-��һ��CH3+��CH3-��Ͼ��ɵõ�CH3CH3
��2��п��һ����Ҫ�Ľ�����п���仯�������Ź㷺��Ӧ�ã�
��ָ��п�����ڱ��е�λ�ã�
��
��
���ڣ���B
��B
�壬ds
ds
��������������п[CH2OH��CHOH��4COO]2Zn��Ŀǰ�г������еIJ�п����д��Zn2+��̬�����Ų�ʽ
1s22s22p63s23p63d10��[Ar]3d10
1s22s22p63s23p63d10��[Ar]3d10
�������Ƿ�����̼ԭ���ӻ���ʽ��sp2��sp3
sp2��sp3
����Zn2+����NH3�������[Zn��NH3��4]2+�����NH3��������
���Է���
���Է���
������Է��ӡ��Ǽ��Է��ӡ�������[Zn��NH3��4]2+�У�Zn2+λ�������������ģ�Nλ����������Ķ��㣬��������ͼ�б�ʾ[Zn��NH3��4]2+��Zn2+��N֮��Ļ�ѧ��������ͼ��ʾп��ij�ǽ���Ԫ��X�γɵĻ����ᄃ��������Zn��Xͨ�����ۼ���ϣ��û�����Ļ�ѧʽΪ
ZnX
ZnX
���û�����ľ����۵�ȸɱ��ߵö࣬ԭ�����ߣ��û�������ԭ�Ӿ��壬���ɱ��Ƿ��Ӿ���
�ߣ��û�������ԭ�Ӿ��壬���ɱ��Ƿ��Ӿ���
��
��������1��A������ȥ��һ����ԭ�Ӳ��ܵõ�CH3+��CH3-�� B��CH3+��CH3-��CH3-�ֱ����6����7����8���۵��ӣ����ǵȵ����壻
C��CH3-��NH3��H3O+������10�����ӣ���Ϊ�ȵ����壻
D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ���
E������CH3-��һ��CH3+��CH3-��϶��ܵõ�CH3CH3��
��2����Zn��ԭ��������30���ڵ������ڣ���B �壬ds ����
����������������̼ԭ�ӣ������������ǻ�ȩ�����ԣ�һ������sp3�ӻ������ǻ���̼����һ������sp2�ӻ���ȩ�����̼����
��ͬ��Ԫ��֮���γɷǼ��Թ��ۼ�����ͬԪ��֮���γɼ��Թ��ۼ�������������������IJ��غϣ�������������������ɵķֲ��Dz����ȵģ����ԳƵģ������ķ���Ϊ���Է��ӣ��Լ��Լ���ϵ�˫ԭ��һ��Ϊ���Է��ӣ��Լ��Լ���ϵĶ�ԭ�ӷ�����ṹ�Գƣ�������ɵ������غϣ���ɷֲ����ȣ���Ϊ�Ǽ��Է��ӣ�[Zn��NH3��4]2+��Zn2+��N֮��Ļ�ѧ������λ����
�ܾ�����п�ĸ���Ϊ4��X�ĸ���Ϊ4����ѧʽΪZnX���û�������������״�ṹ��ԭ�Ӿ��壮
C��CH3-��NH3��H3O+������10�����ӣ���Ϊ�ȵ����壻
D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ���
E������CH3-��һ��CH3+��CH3-��϶��ܵõ�CH3CH3��
��2����Zn��ԭ��������30���ڵ������ڣ���B �壬ds ����
����������������̼ԭ�ӣ������������ǻ�ȩ�����ԣ�һ������sp3�ӻ������ǻ���̼����һ������sp2�ӻ���ȩ�����̼����
��ͬ��Ԫ��֮���γɷǼ��Թ��ۼ�����ͬԪ��֮���γɼ��Թ��ۼ�������������������IJ��غϣ�������������������ɵķֲ��Dz����ȵģ����ԳƵģ������ķ���Ϊ���Է��ӣ��Լ��Լ���ϵ�˫ԭ��һ��Ϊ���Է��ӣ��Լ��Լ���ϵĶ�ԭ�ӷ�����ṹ�Գƣ�������ɵ������غϣ���ɷֲ����ȣ���Ϊ�Ǽ��Է��ӣ�[Zn��NH3��4]2+��Zn2+��N֮��Ļ�ѧ������λ����
�ܾ�����п�ĸ���Ϊ4��X�ĸ���Ϊ4����ѧʽΪZnX���û�������������״�ṹ��ԭ�Ӿ��壮
����⣺��1��A��������ӱ��CH3+��CH3-��CH3-ʱ��ʧȥ�ķֱ����⸺���ӡ������Ӻ����ӣ��ռ乹��Ҳ������ԭ���ķ�����ͬ����A����
B��CH3+��CH3-��CH3-�ֱ����6����7����8���۵��ӣ����ǵȵ����壬����̼ԭ�ӵļ۲���Ӷ�����ͬ���ʿռ乹�Ͳ�ͬ����B����
C��CH3-��NH3��H3O+������10�����ӣ���Ϊ�ȵ����壬���ι��;�Ϊ�����Σ���C��ȷ��
D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ����棬��D��ȷ��
E������CH3-��һ��CH3+��CH3-��Ͽɵõ�CH3CH3����E��ȷ��
��ѡCDE��
��2����Zn��ԭ��������30��������Ų�ʽΪ��1s22s22p63s23p63d104s2���ɵ����Ų�ʽ��֪��Ԫ��Ϊ�������ڣ��ڢ�B��ds�����ʴ�Ϊ���ģ���B��ds��
��Zn2+��̬�����Ų�ʽΪ��1s22s22p63s23p63d10��[Ar]3d10 ����������������̼ԭ�ӣ������������ǻ�ȩ�����ԣ�һ������sp3�ӻ������ǻ���̼����һ������sp2�ӻ���ȩ�����̼�����ʴ�Ϊ��1s22s22p63s23p63d10��[Ar]3d10��sp2��sp3��
����λ��NH3����������������IJ��غϣ�������������������ɵķֲ��Dz����ȵģ����ԳƵģ��Ǽ��Է��ӣ�[Zn��NH3��4]2+��Zn2+��N֮��Ļ�ѧ������λ�����ʴ�Ϊ�����Է��ӣ�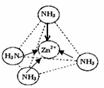 ��
��
�ܾ�����п�ĸ���Ϊ4��X�ĸ���Ϊ4����ѧʽΪZnX���û�������������״�ṹ��ԭ�Ӿ��壬���ɱ��Ƿ��Ӿ��壬�ʸû�������۵�ȸɱ��ߵö࣬�ʴ�Ϊ��ZnX���ߣ��û�������ԭ�Ӿ��壬���ɱ��Ƿ��Ӿ��壮
B��CH3+��CH3-��CH3-�ֱ����6����7����8���۵��ӣ����ǵȵ����壬����̼ԭ�ӵļ۲���Ӷ�����ͬ���ʿռ乹�Ͳ�ͬ����B����
C��CH3-��NH3��H3O+������10�����ӣ���Ϊ�ȵ����壬���ι��;�Ϊ�����Σ���C��ȷ��
D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ����棬��D��ȷ��
E������CH3-��һ��CH3+��CH3-��Ͽɵõ�CH3CH3����E��ȷ��
��ѡCDE��
��2����Zn��ԭ��������30��������Ų�ʽΪ��1s22s22p63s23p63d104s2���ɵ����Ų�ʽ��֪��Ԫ��Ϊ�������ڣ��ڢ�B��ds�����ʴ�Ϊ���ģ���B��ds��
��Zn2+��̬�����Ų�ʽΪ��1s22s22p63s23p63d10��[Ar]3d10 ����������������̼ԭ�ӣ������������ǻ�ȩ�����ԣ�һ������sp3�ӻ������ǻ���̼����һ������sp2�ӻ���ȩ�����̼�����ʴ�Ϊ��1s22s22p63s23p63d10��[Ar]3d10��sp2��sp3��
����λ��NH3����������������IJ��غϣ�������������������ɵķֲ��Dz����ȵģ����ԳƵģ��Ǽ��Է��ӣ�[Zn��NH3��4]2+��Zn2+��N֮��Ļ�ѧ������λ�����ʴ�Ϊ�����Է��ӣ�
 ��
���ܾ�����п�ĸ���Ϊ4��X�ĸ���Ϊ4����ѧʽΪZnX���û�������������״�ṹ��ԭ�Ӿ��壬���ɱ��Ƿ��Ӿ��壬�ʸû�������۵�ȸɱ��ߵö࣬�ʴ�Ϊ��ZnX���ߣ��û�������ԭ�Ӿ��壬���ɱ��Ƿ��Ӿ��壮
���������⿼���жϼ��ӻ����ӵĹ��͡����Է��ӡ������ļ��㡢ԭ�ӹ���ӻ���ʽ���ӻ������жϡ��������͵��жϵȣ��ѶȽϴ����վ����ļ����ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
�����Ŀ
 ��R1��R2��R3����������
��R1��R2��R3����������

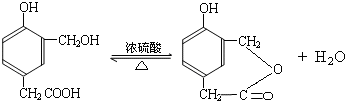
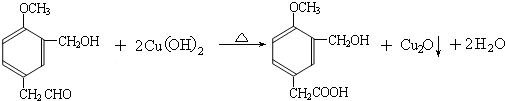
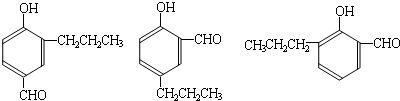
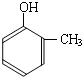 ���ĺϳ�
���ĺϳ�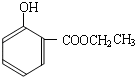 ·�ߣ��úϳ�·������ͼ��ʾ����ע����Ӧ��������
·�ߣ��úϳ�·������ͼ��ʾ����ע����Ӧ��������
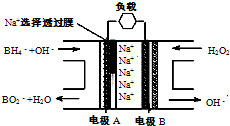 ��2010?������ģ�⣩ֱ��NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ�����������ϲ���Pt/C���������ϲ���MnO2�����йظõ�ص�˵����ȷ����
��2010?������ģ�⣩ֱ��NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ�����������ϲ���Pt/C���������ϲ���MnO2�����йظõ�ص�˵����ȷ����