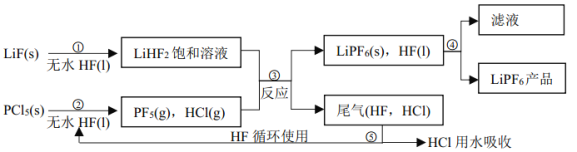
��Ŀ����
����Ŀ����Al2��SO4��3��AlCl3�Ļ����Һ����μ���1mol/L Ba��OH��2��Һ������������Ba��OH��2��Һ����������ó��������ʵ����Ĺ�ϵ��ͼ������˵������ȷ������ ��
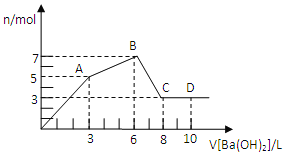
A��ͼ��C����Ԫ�ش�����ʽ��AlO2��
B����D����Һ��ͨ��C02���壬����������ɫ����
C��ԭ���Һ��c[Al2��SO4��3]��c��AlCl3��=1��2
D��OA�η�Ӧ�����ӷ���ʽΪ��3Ba2++2Al3++8OH-+3SO4=BaSO4��+2AlO2-+4H2O
���𰸡�D
��������
�����������Al2��SO4��3 �� AlCl3�Ļ����Һ��Ba��OH��2��Һ��Ӧ��ʵ����Al3+��OH-��Ba2+��SO42-֮������ӷ�Ӧ�����£�Ba2++SO42-�TBaSO4����Al3++3OH-�TAl��OH��3����Al��OH��3+OH-�TAlO2-+2H2O������1molAl2��SO4��3��SO42-��ȫ����������Ba��OH��2��Ϊ3mol���ṩ6molOH-��1molAl2��SO4��3�к���2molAl3+���ɷ�ӦAl3++3OH-�TAl��OH��3����֪��2molAl3+��ȫ��������Ҫ6molOH-���ʣ�����㵽A�㣬������Ϊ������������������ǡ�÷�����Ӧ�������ᱵ����������������A��ʱSO42-��ȫ������A-BΪ�Ȼ��������������ķ�Ӧ��B��ʱ��Һ��Al3+��ȫ�������������������ֵ����Һ������ΪBaCl2��B-CΪ��������������������Ӧ��C��ʱ����������ȫ�ܽ⡣A��C��ʱ����������ȫ�ܽ⣬ת��Ϊƫ�����Σ���C����Ԫ�ش�����ʽ��AlO2-����A��ȷ��B��D�����Һ�к���Ba2+��AlO2-��ͨ�������̼��������̼�ᱵ������������������B��ȷ��C��ǰ3LBa��OH��2��Һ����Һ��Al2��SO4��3��Ӧ����3L-6LΪBa��OH��2��Һ����Һ��AlCl3��Ӧ���������ĵ��������������ʵ������Ϊ3L��1mol/L=3mol�����������ᱵ��֪3n[Al2��SO4��3]=n[Ba��OH��2]����n[Al2��SO4��3]=1mol�����Ȼ���������������������������֪3n��AlCl3��=2[Ba��OH��2]=6mol����n��AlCl3��=2mol����ԭ��Һ��ԭ���Һ��c[Al2��SO4��3]��c��AlCl3��=1��2����C��ȷ��D��OA��ʵ��Ϊ����������������ǡ�÷�����Ӧ�������ᱵ������������������Ӧ���ӷ���ʽΪ��3Ba2++2Al3++6OH-+3SO42-=3BaSO4��+2Al��OH��3������D����ѡD��