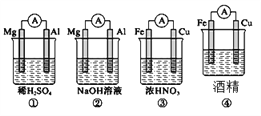
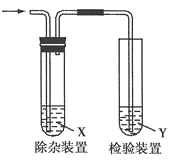
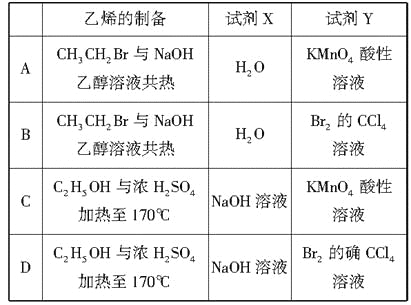
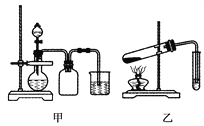
题目内容
【题目】某致病细菌分泌的外毒素,无色,细针状结晶,对小鼠和人体有很强的毒性,可引起流涎、呕吐、便血、痉挛等症状,以致死亡。该外毒素为环状肽,其结构简式如图所示:

请据图分析回答:
(1)该化合物中含有游离的___________个氨基,___________个羧基。
(2)该化合物是由___________个氨基酸组成的,区别这些氨基酸的种类依靠的是其结构中的___________。
(3)组成该化合物的氨基酸有_________种,其中有_______个氨基酸的R基相同,这个R基是________。
(4)该化合物为___________肽化合物,含有___________个肽键。
(5)填写虚线框内结构的名称:A.______________________, B.______________________。
【答案】 0 0 7 R基 5 3 —CH2 环状七 7 肽键 R基
【解析】试题分析:本题考查氨基酸的结构通式的解读、多肽链(环状多肽)的合成特点和有关游离的氨基和羧基的计算
(1)由于该多肽是环肽链,首尾氨基酸羧基和氨基脱水缩合,再加上R基上没有游离的氨基和游离的羧基,该化合物没有游离的氨基和游离的羧基。(2)由于该化合物存在七个R基,所以该化合物有7个氨基酸组成,区别氨基酸的种类依靠结构的R基种类。(3)由图知,有5种不同的R基,意味着组成该化合物的氨基酸有5种,其中3个氨基酸的R基都是—CH3 。(4)该化合物的环肽链,由7个氨基酸组成,叫做环状7肽化合物,含有7个肽键。(5)A表示肽键,B表示R基

【题目】在2 L的恒容密闭容器中充入A(g)和B(g),发生反应:A(g)+B(g) ![]() 2C(g)+D(s) ΔH=a kJ·mol-1实验内容和结果分别如表和图所示。下列说法正确的是( )
2C(g)+D(s) ΔH=a kJ·mol-1实验内容和结果分别如表和图所示。下列说法正确的是( )
实验 序号 | 温度 | 起始物质的量 | 热量 变化 | |
A | B | |||
Ⅰ | 600℃ | 1 mol | 3 mol | 96 kJ |
Ⅱ | 800℃ | 1.5 mol | 0.5 mol | ____ |
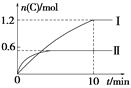
A. 实验Ⅰ中,10 min内平均速率v(B)=0.06 mol·L-1·min-1
B. 上述方程式中a=160
C. 600℃时,该反应的平衡常数是0.45
D. 向实验Ⅱ的平衡体系中再充入0.5 mol A和1.5 mol B,A的转化率增大