��Ŀ����
����Ŀ����ï���ڳ�����Ϊ�Ȼ�ɫ��ĩ����������ζ���������ܡ����̡���ȼ���Ӽ��ȡ��������з����ϳɣ�
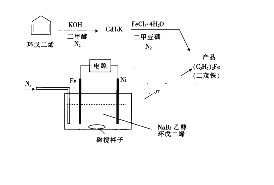
�ش��������⣺
(1)�����£������ϩ�Զ��������ʽ���ڡ�ijʵ��С��Ҫ�õ������ϩ�����Ƚ������ϩ�������ۣ���֪��
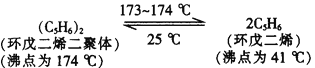
�ٷ���õ������ϩ�IJ���Ϊ_____________________(���������)��
���ɻ����ϩ����C5H5K�Ļ�ѧ����ʽΪ___________________��
(2)�����������FeCl2��4H2O��ͨ�����в����Ƶá�
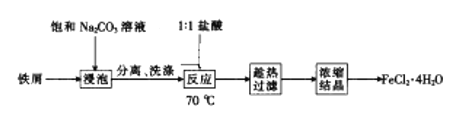
���ñ���Na2CO3��Һ����������м��Ŀ����_____________________________________��
�ڲ��������ȹ���������ԭ����________________________________________________��
(3)�õ�ⷨ�Ʊ���ï��ʱ��
�ٲ���ͨ��N2��Ŀ����__________________________��
��Ni�缫���Դ��____________(��������������������)�������������������ĵ缫��ӦʽΪ__________________�������ϵ�·��ÿת��1 mol���ӣ��õ���Ʒ������Ϊ____��
���𰸡����� C5H6+KOH ![]() C5H5K+H2O ����м��������۳�ȥ ��ֹ���¹���ʱFeCl2��4H2O�ᾧ��������ʧ ʹ��������������½��У���ֹFe2+������ ���� Fe-2e-=Fe2+ 93g
C5H5K+H2O ����м��������۳�ȥ ��ֹ���¹���ʱFeCl2��4H2O�ᾧ��������ʧ ʹ��������������½��У���ֹFe2+������ ���� Fe-2e-=Fe2+ 93g
��������
��1���ٻ��ܵ��л���ɸ��ݷе㲻ͬ����������ķ������з��룻
�ڴ�����ͼ�и�����Ϣ����
��2���Ʊ���ˮFeCl2������Na2CO3��Һ����������̼������Һ�ļ��Գ�ȥ����������ۣ����˵õ���м��ϴ�ӡ���ɣ����������Ӧ����м������ȷ����Ӧ���Һ���в���Fe3+�������Ȼ����������ȹ��ˣ������Ȼ���������������ҺŨ���ᾧFeCl24H2O���Ȼ�����Ϊǿ�������Σ�ˮ�⣬�����Ȼ������������ˮ�õ���ˮ�Ȼ�������
��3���ٶ�ï��������+2�ۣ��ױ�����������������ͨ�뵪���ž�װ���п�����ʹ��������������½��У���ֹFe2+��������
����������ͼ���Կ�����Fe�����ص�����ʧ���ӷ���������Ӧ��Ni�缫���ӵ�Դ�ĸ����������������ݵ��ԭ����������
��1�� �ٸ��������Ϣ��֪,�����ϩ������( �е�Ϊ174��)�������ϩ(�е�Ϊ41��)������õ������ϩ�����������ߵķе����ϴ�������ķ������з��룬
�ʴ�Ϊ������
�ڴ�����ͼ�п��Կ������ɻ����ϩ����C5H5K�Ļ�ѧ����ʽΪ��C5H6+KOH ![]() C5H5K+H2O��
C5H5K+H2O��
��2���������ڼ���������ˮ�⣬����Na2CO3��Һ����������̼������Һ�ļ��Գ�ȥ����������ۣ�
��һ��������ܽ�����¶ȵ����߶�������˳��ȹ��˿ɷ�ֹ���¹���ʱFeCl2��4H2O�ᾧ��������ʧ��
��3�� �ٶ�ï��������+2�ۣ��ױ�����������������ͨ�뵪���ž�װ���п�������ֹʵ��������������ӱ�������
�ʴ�Ϊ���ž�װ���ڿ�����
��Fe�����ص�����ʧ���ӷ���������Ӧ����缫��ӦʽΪ��Fe-2e-=Fe2+��Ni�����ص����������ӵ�Դ�ĸ������ڵ������У������ϵ�·��ÿת��1 mol���ӣ����ɵ�Fe2+�����ʵ���Ϊ0.5mol����õ��Ķ�ï�������ʵ���Ϊ0.5mol��������Ϊ0.5mol��186g/mol=93g��

����Ŀ��A��B��C��D��E�����ֶ�����Ԫ�ء���֪�����ǵ�ԭ��������������A��Ԫ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Bԭ���������������������������2��C��E������Ԫ�أ�D��E��ԭ������֮��Ϊ30����D������������������ȡ��ס��ҡ������������������γɵĻ�������м����к���18�����ӡ�
������� | �� | �� | �� | �� |
�������и�Ԫ�� ԭ�Ӹ����� | A��C 1:1 | B��A 1:4 | D��E 1:3 | B��E 1:4 |
��ش��������⣺
��1��Ԫ��E�����ڱ��е�λ��Ϊ___________________________��
��2����D�ĵ��ʷŵ�NaOH��Һ�У���Ӧ�Ļ�ѧ����ʽΪ��_______________________��
��3���õ���ʽ��ʾ���γɹ��̣�_________________________��
��4�����ܱ������г���BC2��BC���ҵĻ�����干mg������������Na2O2,����������õ��ȼ����Ӧ��ȫ����ù�����������mg����BC2���ҵ������Ϊ________________��
��5����200mL MgCl2�ͱ��Ļ����Һ������c(Mg2+)�� 0.2 mol�� L-1��c(Cl-)�� 1.3mol��L-1��ҪʹMg2��ȫ��ת��Ϊ�������������������Ҫ4 mol��L-1 NaOH ��Һ������ǣ�______��
����Ŀ��������������������ȣ����ɰ��͵����϶��á����ǵĽṹ���£�

�������м������ݣ���Ӧ8P4(s)��3S8(s)=8P4S3(g)����HΪ(����)
��ѧ�� | P��P | S��S | P��S |
����/kJ��mol��1 | a | b | c |
A.24(a��b��2c) kJ��mol��1B.(32a��24b��24c) kJ��mol��1
C.(48c��24a��24b) kJ��mol��1D.(8a��3b��3c) kJ��mol��1



