��Ŀ����
12�����й�����Һ�����ӵ�˵������ȷ���ǣ�������| A�� | 0.1mol•L-1��Na2CO3��Һ������Ũ�ȹ�ϵ��c��Na+���T2c��CO32-��+2c��HCO3-��+2c��H2CO3�� | |
| B�� | �����£�����������Һ�е������ʹ��Һ��pH=7��������Һ�д����ѹ��� | |
| C�� | 0.2mol•L-1��HCl��0.1 mol•L-1��NH3•H2O�������Ϻ���Һ�е�����Ũ�ȹ�ϵ��c��Cl-����c ��NH4+����c��H+����c ��OH-�� | |
| D�� | 0.1mol•L-1��NaHS��Һ������Ũ�ȹ�ϵ��c��S2-��+c��OH-���Tc��H+��+c��H2S�� |
���� A��Na2CO3��Һת�����������غ㣬��Ԫ�����ʵ���Ϊ̼Ԫ�����ʵ�����2����
B���������������ǡ�÷�Ӧ���ɴ�������Һ�ʼ��ԣ�����Һ��������Ҫ���������
C��0.2mol•L-1��HCl��0.1 mol•L-1��NH3•H2O�������Ϻ���Һ����HCl��NH4Cl�Ļ����Һ��笠�����ˮ���������Ũ�ȴ�С��
D��������Һ�������غ�͵���غ��ʽ�����жϣ�
��� �⣺A��0.1mol•L-1��Na2CO3��Һ�������غ�ʽΪc��Na+��=2c��CO32-��+2c��HCO3-��+2c��H2CO3������A��ȷ��
B���������������ǡ�÷�Ӧ���ɴ�������Һ�ʼ��ԣ�����Һ��������Ҫ����������ſ���ʹ��Һ��ʾ���ԣ���B��ȷ��
C��0.2mol•L-1��HCl��0.1 mol•L-1��NH3•H2O�������Ϻ���Һ����HCl��NH4Cl�Ļ����Һ����Һ��笠�����ˮ�⣬������Ũ�ȴ���笠�����Ũ�ȣ�c��Cl-����c��H+����c ��NH4+����c ��OH-������C����
D��0.1mol•L-1��NaHS��Һ�е���غ㣬c��Na+��+c��H+��=c��OH-��+c��HS-��+2c��S2-���������غ�c��Na+��=c��HS-��+c��S2-��+c��H2S�����������õ�c��S2-��+c��OH-���Tc��H+��+c��H2S������D��ȷ��
��ѡC��
���� ���⿼���˵������Һ�е���غ㡢�����غ㡢����Ũ�ȴ�С�Ƚϣ����շ����ͼ��㷽�������Ӵ�����ʽ�ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ҵ�ƾ��к����Ҵ��ͼ״� | B�� | ��ú̿��ʯ���У����ĺ������ϸ� | ||
| C�� | ����ˮ���к���������� | D�� | ��Ȼ��֬�к��и�֬��������� |
| A�� | ���ˮ�еμӱ���FeCl3��Һ�Ʊ�Fe��OH��3���壺Fe3++3H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3��+3H+ | |
| B�� | Fe��OH��3��������Fe��OH��3+3H+�TFe3++3H2O | |
| C�� | ��С�մ�����θ����ࣺHCO3-+H+�TCO2��+H2O | |
| D�� | ��Pt�缫��ⱥ���Ȼ�þ��Һ��2Cl-+2H2$\frac{\underline{\;���\;}}{\;}$2OH-+H2��+Cl2�� |
| A�� | �ɵ�ع������ | B�� | �������� | ||
| C�� | ֲ��ͨ�������������̫���� | D�� | ��ѩ�ڻ� |
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��������ˮ����NaBr��Һ�У���������CCl4������ | �Ƚ������嵥�ʵ�������ǿ�� |
| B | �ֱ���2ȥ�Թ��м���3ml��5%��H2O2��Һ��һ֧����5�����ҵ���ˮ�У���һ֧����40�����ҵ���ˮ�У��۲� | �Ƚ��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�� |
| C | ����Һ�еμ������ữ���Ȼ�����Һ | ����ij��Һ���Ƿ�һ����SO42- |
| D | ��ʵ�����Ƶõ�CO2����ͨ������NaHCO3��Һ��Ũ���� | ��ȥCO2�е�HCl��H2O���� |
| A�� | A | B�� | B | C�� | C | D�� | D |

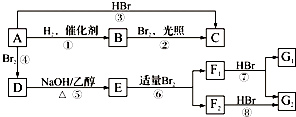
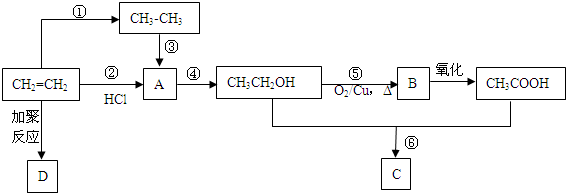
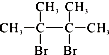 ��F1��F2��Ϊͬ���칹�壬�ӳ�HBrʱF1�����ּӳɲ��F2ֻ��һ�ּӳɲ��G1��G2�ֱ�Ϊͬ���칹�壮�ݴ�����
��F1��F2��Ϊͬ���칹�壬�ӳ�HBrʱF1�����ּӳɲ��F2ֻ��һ�ּӳɲ��G1��G2�ֱ�Ϊͬ���칹�壮�ݴ�����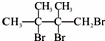 ��
��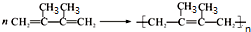
 ��
�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O��