��Ŀ����
���л���ѧ������
��1��������������Ĺ�����������Ӧ�����������������
��������CH2 =CH2��CH3 CH3 ��Ӧѡ�� __ ������ĸ����
a��NaOH��Һ b����ˮ c��������Һ
��������HCHO��HCOOH��Ӧѡ�� _ (����ĸ)��
a��KMnO4��Һ b��������Һ c��Na2CO3��Һ
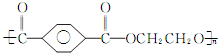
��������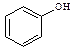 ��
�� ��Ӧѡ�� ___ (����ĸ)��
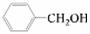
��Ӧѡ�� ___ (����ĸ)��
a��FeCl3��Һ b��NaOH��Һ c��AgNO3��Һ
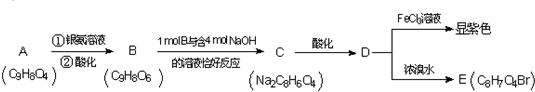
��2�����л���ѧ�У�ͬ���칹���ձ����������ʽΪC4H9OH���л��ﹲ��
�֡����У�һ���л���ͨ����ȥ��Ӧ��ת��Ϊ2-��ϩ����д������ȥ��Ӧ�Ļ�ѧ����ʽ  ����һ���л���ĺ˴Ź�������ͼ��1H�˴Ź�����ͼ������ʾһ���壬��д�����л���Ľṹ��ʽ���£� ��
����һ���л���ĺ˴Ź�������ͼ��1H�˴Ź�����ͼ������ʾһ���壬��д�����л���Ľṹ��ʽ���£� ��
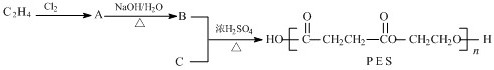
��3��A��ʯ���ѽ����ijɷ�֮һ��A��ijһͬϵ��E�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�C6H12O2����ϳ�·������ͼ��ʾ��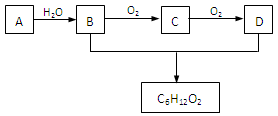
�ش��������⣺
��A�Ľṹ��ʽΪ��______________��
��B��D�����еĹ��������Ʒֱ�Ϊ_______________��_____________��
��д��B��ͬ����ͬ���칹��Ľṹ��ʽ��___________________��
��д��B��C�Ļ�ѧ����ʽ��_______________________ __________________��
__________________��
15��
��1����b��1�֣�  ��c��1�֣� ��a��1�֣�
��c��1�֣� ��a��1�֣�
��2��4��1�֣� H3CCH2CH(OH)CH3 CH3CH=CHCH3+H2O ��2�֣�
CH3CH=CHCH3+H2O ��2�֣�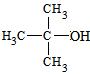 ��2�֣�
��2�֣�
��3����CH3CH=CH2��1�֣� ���ǻ����Ȼ���2�֣� ��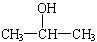 ��2�֣�[��Դ:ѧ+��+��]��2CH3CH2CH2OH+O2
��2�֣�[��Դ:ѧ+��+��]��2CH3CH2CH2OH+O2 2CH3CH2CHO+2H2O��2�֣�
2CH3CH2CHO+2H2O��2�֣�
����

 ��У����ϵ�д�
��У����ϵ�д�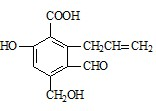



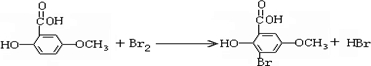

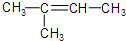
 ��ʾ�ķ���ʽ
��ʾ�ķ���ʽ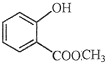 �к��еĹ����ŵ�����Ϊ
�к��еĹ����ŵ�����Ϊ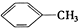 ��
�� ��
��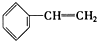 ��C2H4 ��CH2=CH-C=CH2 ��C3H6 ��
��C2H4 ��CH2=CH-C=CH2 ��C3H6 �� ��
��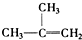
 ��
��

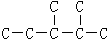 ����������һ�������
����������һ�������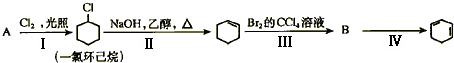
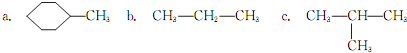
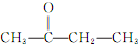 ��
��