��Ŀ����
B�����л���ѧ��������1�����ݽṹ���л�����з��࣬�����ڶ������ʵ����գ�
�������л�������ֱ��������
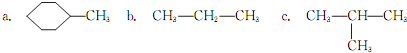
�������л���������֬����
a��Ӳ֬������� b������������� c�����������
�������л���������Ȼ�߷��ӻ��������
a���������� b��Ӳ֬���� c��������
��2��������X�Ľṹ��ʽΪ
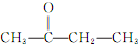 ��
����X��1H�˴Ź�����ͼ����
����X��Ϊͬ���칹�壬���ܷ���������Ӧ�Ľṹ��
����X��Ϊͬ���칹���һ���л�����ӵĽṹ��ʽΪCHCH2H2CCH2OH����д���䷢����ȥ��Ӧ�Ļ�ѧ����ʽ��
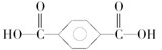
��3��PET�ǵ��ڵ���Ҫ�ɷ֣�����������ƿ���Ŵ��ͽ�Ƭ��Ƭ���ȣ���ṹ��ʽ��ͼ��
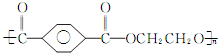
�ٸø߷��Ӳ����������ֵ���ͨ��
�ڼ����������ֵ���ķ���Ϊ
��������1����ֱ������ָ����֧����������
����֬Ϊ��֬����ĸ�������
����Ȼ�߷��ӻ�������Ҫָ���ۡ���ά�ء������ʺ���Ȼ�ȣ�
��2���ٺ˴Ź��������з�ֵ�������л�������ԭ�ӵ���������
���ܷ���������Ӧ�����ʺ���ȩ�������ţ�ͬ���칹���Ǽ�����ͬ�ķ���ʽ����ͬ�ṹ�Ļ����
�۴�������ȥ��Ӧ�Ľṹ�ص㣺���ǻ�����̼����̼������ԭ�Ӳ��ܷ�����ȥ��Ӧ���γɲ����ͼ���
��3���ٷ������м䣨���ڶ��ϣ�����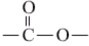 ���������ṹ�ĸ߾���䵥���Ϊ���֣�
���������ṹ�ĸ߾���䵥���Ϊ���֣�
��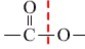 �Ͽ����ʻ��ϼ��ǻ�����ԭ���ϼ���ԭ�Ӽ��ø߾��ﵥ�壻
�Ͽ����ʻ��ϼ��ǻ�����ԭ���ϼ���ԭ�Ӽ��ø߾��ﵥ�壻
�����������ԣ������������ԣ��ݴ˼���
����֬Ϊ��֬����ĸ�������
����Ȼ�߷��ӻ�������Ҫָ���ۡ���ά�ء������ʺ���Ȼ�ȣ�
��2���ٺ˴Ź��������з�ֵ�������л�������ԭ�ӵ���������
���ܷ���������Ӧ�����ʺ���ȩ�������ţ�ͬ���칹���Ǽ�����ͬ�ķ���ʽ����ͬ�ṹ�Ļ����
�۴�������ȥ��Ӧ�Ľṹ�ص㣺���ǻ�����̼����̼������ԭ�Ӳ��ܷ�����ȥ��Ӧ���γɲ����ͼ���
��3���ٷ������м䣨���ڶ��ϣ�����
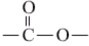 ���������ṹ�ĸ߾���䵥���Ϊ���֣�
���������ṹ�ĸ߾���䵥���Ϊ���֣���
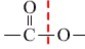 �Ͽ����ʻ��ϼ��ǻ�����ԭ���ϼ���ԭ�Ӽ��ø߾��ﵥ�壻
�Ͽ����ʻ��ϼ��ǻ�����ԭ���ϼ���ԭ�Ӽ��ø߾��ﵥ�壻�����������ԣ������������ԣ��ݴ˼���
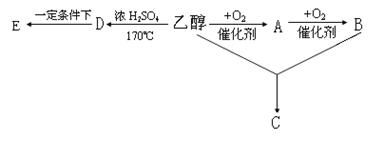
����⣺��1����ֱ������ָ����֧����������aΪ��״���������ϣ�c�м�֧������b���ϣ�
�ʴ�Ϊb��
����֬Ϊ��֬����ĸ������������������ڸ�֬���ᣬ��bc��������֬����ѡa���ʴ�Ϊ��a��
����Ȼ�߷��ӻ�������Ҫָ���ۡ���ά�ء������ʺ���Ȼ�ȣ�����������Ӳ֬��������С���ӣ�������������Ȼ�߷��ӣ���ѡc��
�ʴ�Ϊ��c��
��2���ٺ˴Ź��������з�ֵ�������л�������ԭ�ӵ������������л�������3����ԭ�ӣ�����3�����շ壻
�ʴ�Ϊ��3��
���ܷ���������Ӧ�����ʺ���ȩ�������ţ����������֣�CH3CH2CH2CHO��CH3CH��CH3��CHO��
�ʴ�Ϊ��2��
�۴�������ȥ��Ӧ�Ľṹ�ص㣺���ǻ�����̼����̼������ԭ�Ӳ��ܷ�����ȥ��Ӧ���γɲ����ͼ�����Ӧ����ʽΪ��
CH2�TCHCH2CH2OH
CH2�TCH-CH�TCH2+H2O��
�ʴ�Ϊ��CH2�TCHCH2CH2OH
CH2�TCH-CH�TCH2+H2O��
��3����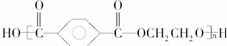 ����
���� ��
��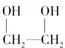 �������۷�Ӧ���ɣ�
�������۷�Ӧ���ɣ�
�ʴ�Ϊ�����۷�Ӧ ��
��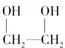 ��
��
�ڶԱ������������ԣ���ʹָʾ����ɫ���������ͨ�ԣ��Ҷ��������ԣ���ʹָʾ����ɫ���ʿ���ָʾ�����𣬻���̼�����ơ�������ͭ�ȼ��𣬶Ա���������̼�����ƻ�������ͭ��Ӧ������ų�������ܽ⣬���Ҷ�������Ӧ��
�ʴ�Ϊ�����ָʾ������̼�����ơ�������ͭ�ȣ���
�ʴ�Ϊb��
����֬Ϊ��֬����ĸ������������������ڸ�֬���ᣬ��bc��������֬����ѡa���ʴ�Ϊ��a��
����Ȼ�߷��ӻ�������Ҫָ���ۡ���ά�ء������ʺ���Ȼ�ȣ�����������Ӳ֬��������С���ӣ�������������Ȼ�߷��ӣ���ѡc��
�ʴ�Ϊ��c��
��2���ٺ˴Ź��������з�ֵ�������л�������ԭ�ӵ������������л�������3����ԭ�ӣ�����3�����շ壻
�ʴ�Ϊ��3��
���ܷ���������Ӧ�����ʺ���ȩ�������ţ����������֣�CH3CH2CH2CHO��CH3CH��CH3��CHO��
�ʴ�Ϊ��2��
�۴�������ȥ��Ӧ�Ľṹ�ص㣺���ǻ�����̼����̼������ԭ�Ӳ��ܷ�����ȥ��Ӧ���γɲ����ͼ�����Ӧ����ʽΪ��
CH2�TCHCH2CH2OH
| ŨH2SO4 |
| �� |
�ʴ�Ϊ��CH2�TCHCH2CH2OH
| ŨH2SO4 |
| �� |
��3����
 ����
���� ��
��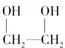 �������۷�Ӧ���ɣ�
�������۷�Ӧ���ɣ��ʴ�Ϊ�����۷�Ӧ
 ��
��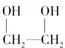 ��
���ڶԱ������������ԣ���ʹָʾ����ɫ���������ͨ�ԣ��Ҷ��������ԣ���ʹָʾ����ɫ���ʿ���ָʾ�����𣬻���̼�����ơ�������ͭ�ȼ��𣬶Ա���������̼�����ƻ�������ͭ��Ӧ������ų�������ܽ⣬���Ҷ�������Ӧ��
�ʴ�Ϊ�����ָʾ������̼�����ơ�������ͭ�ȣ���
���������⿼�����л���ķ��ࡢ�ṹ�����ʡ�ͬ���칹����д���߾��ﵥ����жϵȣ��е��Ѷȣ�ע�ⷲ�����м䣨���ڶ��ϣ�����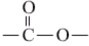 ���������ṹ�ĸ߾���䵥���Ϊ���֣���
���������ṹ�ĸ߾���䵥���Ϊ���֣��� 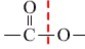 �Ͽ����ʻ��ϼ��ǻ�����ԭ���ϼ���ԭ�Ӽ��ø߾��ﵥ�壮
�Ͽ����ʻ��ϼ��ǻ�����ԭ���ϼ���ԭ�Ӽ��ø߾��ﵥ�壮
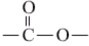 ���������ṹ�ĸ߾���䵥���Ϊ���֣���
���������ṹ�ĸ߾���䵥���Ϊ���֣��� 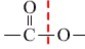 �Ͽ����ʻ��ϼ��ǻ�����ԭ���ϼ���ԭ�Ӽ��ø߾��ﵥ�壮
�Ͽ����ʻ��ϼ��ǻ�����ԭ���ϼ���ԭ�Ӽ��ø߾��ﵥ�壮
��ϰ��ϵ�д�
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ

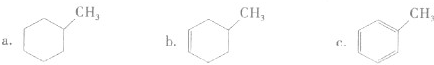
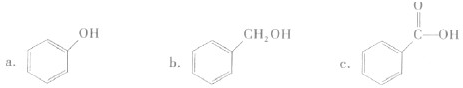
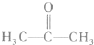


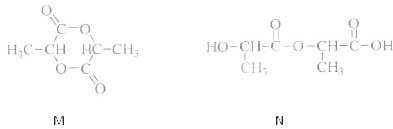
 ����
���� ���ĸ�����Ϊ
���ĸ�����Ϊ 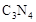 �γɵ�ԭ�Ӿ��壬�ṹ����
�γɵ�ԭ�Ӿ��壬�ṹ����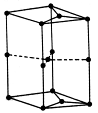
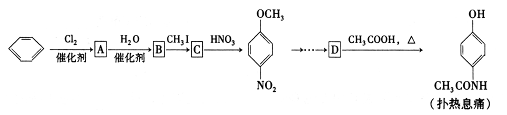
 C�ķ�Ӧ����Ϊ ��D�й����ŵ�����Ϊ ��
C�ķ�Ӧ����Ϊ ��D�й����ŵ�����Ϊ ��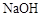 ��Һ��Ӧ������������
��Һ��Ӧ������������ ����ش�
����ش�