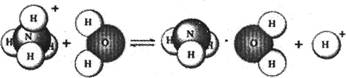
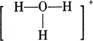
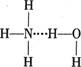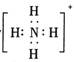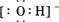��Ŀ����
��1���ڱ����Ȼ�����Һ�м���̼��Ʒ�ĩ������̼������ܽ⣬ͬʱ��������������
��____________________________________________________________________________��
��____________________________________________________________________________��
�����ӷ���ʽ��ʾ�������������ԭ��___________________________________________
____________________________________________________________________________��
��2���ڱ����Ȼ�����Һ�м���һ�ֵ��ʣ�Ҳ�����������������ķ�Ӧ�����ֵ�����________��
��3�������ʵ���֮��Ϊ0.1mol��FeCl3��AlCl3�Ļ����Һ�м���90mL4mol��L-1��NaOH��Һ��ʹ���ַ�Ӧ������ԭ��Һ��Al3+���ʵ�����Fe3+��Al3+�����ʵ���֮��Ϊx��
�ٵ�x=0.4ʱ����Һ�в����ij�����_____________�������ʵ���Ϊ_____________��
�ڵ�����ֻ��Fe(OH)3ʱ��x��ȡֵ��ΧΪ_____________��
��____________________________________________________________________________��
��____________________________________________________________________________��
�����ӷ���ʽ��ʾ�������������ԭ��___________________________________________
____________________________________________________________________________��
��2���ڱ����Ȼ�����Һ�м���һ�ֵ��ʣ�Ҳ�����������������ķ�Ӧ�����ֵ�����________��
| A���� | B���� | C��þ | D��ͭ |
�ٵ�x=0.4ʱ����Һ�в����ij�����_____________�������ʵ���Ϊ_____________��
�ڵ�����ֻ��Fe(OH)3ʱ��x��ȡֵ��ΧΪ_____________��
������ɫ��ζ�������ɢ���Һ��ɫ����������ɫ����Fe3++3H2O Fe(OH)3+3H+CaCO3+2H+====Ca2++CO2��+H2O��2��C��3����Fe(OH)30.06mol��0��x��0.6
Fe(OH)3+3H+CaCO3+2H+====Ca2++CO2��+H2O��2��C��3����Fe(OH)30.06mol��0��x��0.6
 Fe(OH)3+3H+CaCO3+2H+====Ca2++CO2��+H2O��2��C��3����Fe(OH)30.06mol��0��x��0.6
Fe(OH)3+3H+CaCO3+2H+====Ca2++CO2��+H2O��2��C��3����Fe(OH)30.06mol��0��x��0.6(1)Fe3++3H2O Fe(OH)3+3H+2H++CaCO3====Ca2++H2O+CO2�����ԣ�ƽ�������ƶ�����Һ��ɫ����������ɫ������ͬʱ����CO2��
Fe(OH)3+3H+2H++CaCO3====Ca2++H2O+CO2�����ԣ�ƽ�������ƶ�����Һ��ɫ����������ɫ������ͬʱ����CO2��
(2)Mg����H+��Ӧ
(3)��x=0.4����n(AlCl3)=0.04mol��n(FeCl3)=0.06mol��n(NaOH)=4mol��L-1��0.09L=0.36mol
n��Fe(OH)3��=0.06mol��Fe3+����NaOH��0.06mol��3=0.18mol
n(NaOH)����n(AlCl3)=��0.36-0.18��mol��0.04mol=9��2
����Al3+ȫ��ת��Ϊ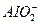 ,������
,������
��n(AlCl3)=0.1xmoln(FeCl3)=0.1(1-x)mol������ֻ��Fe(OH)3����Al3+ȫ��ת��Ϊ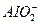 �����ݵ���غ�ã�
�����ݵ���غ�ã�
0.1x+3��0.1x+3��0.1(1-x)��0.36����x��0.6
����0��x��0.6��
 Fe(OH)3+3H+2H++CaCO3====Ca2++H2O+CO2�����ԣ�ƽ�������ƶ�����Һ��ɫ����������ɫ������ͬʱ����CO2��
Fe(OH)3+3H+2H++CaCO3====Ca2++H2O+CO2�����ԣ�ƽ�������ƶ�����Һ��ɫ����������ɫ������ͬʱ����CO2��(2)Mg����H+��Ӧ
(3)��x=0.4����n(AlCl3)=0.04mol��n(FeCl3)=0.06mol��n(NaOH)=4mol��L-1��0.09L=0.36mol
n��Fe(OH)3��=0.06mol��Fe3+����NaOH��0.06mol��3=0.18mol
n(NaOH)����n(AlCl3)=��0.36-0.18��mol��0.04mol=9��2
����Al3+ȫ��ת��Ϊ
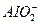 ,������
,��������n(AlCl3)=0.1xmoln(FeCl3)=0.1(1-x)mol������ֻ��Fe(OH)3����Al3+ȫ��ת��Ϊ
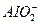 �����ݵ���غ�ã�
�����ݵ���غ�ã�0.1x+3��0.1x+3��0.1(1-x)��0.36����x��0.6
����0��x��0.6��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
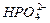
 H++
H++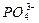 ����ʹ��Һ��c(
����ʹ��Һ��c( )��ͬ��������Һ��NH4Cl��(NH4)2SO4?��NH4HSO4?��NH4HCO3,�����ʵ���Ũ���ɴ�С��˳���ǣ���
)��ͬ��������Һ��NH4Cl��(NH4)2SO4?��NH4HSO4?��NH4HCO3,�����ʵ���Ũ���ɴ�С��˳���ǣ���