��Ŀ����
9��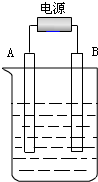 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫϡ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫϡ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺��1��A�缫�ǽӵ�Դ�ĸ�����B�缫�ķ�Ӧʽ4OH--4e-=2H2O+O2����
��2��ԭ��Һ�����ʵ���Ũ����0.05mol/L��������Һ��pHΪ1����������ǰ����Һ��������䣩
��3���������ʵ��ȷ��ԭ��Һ�п���������������ӣ�Ҫ��������ֿ��ܵļ��裬�ֱ�д����֤�����ּ���IJ������衢ʵ�������ʵ����ۣ�
����ټ���ԭ��Һ�е������������������ӣ�ȡ�������Һ�������м���BaCl2��Һ�����а�ɫ�������ɣ���ԭ��Һ����������������SO42-
����ڼ���ԭ��Һ�е������������������ӣ�ȡ�������Һ�������м���ͭ���ȣ���ͭ�ܽ⣬������ɫ�������ɣ����ڿ����б�Ϊ����ɫ����ԭ��Һ����������������NO3-��
���� ��1���͵�Դ�ĸ����������ǵ��ص�����������ԭ��Ӧ���͵�Դ�������������ǵ��ص���������������Ӧ��
��2��������������ͭ��������1.6g�����ݵ缫��Ӧ��������ͭ�����ʵ����Լ���Һ�������ӵ����ʵ�������������Ũ�ȵ������ʵ��������֮�ȼ������ʵ���Ũ���Լ�pH��
��3������Һ��һ���Ǻ�ͭ���ӵĿ����Ե�����Һ�����Կ���������ͭ����������ͭ���������ӵ��������ӷ�Ӧ��������������Լ���������ӵĴ��ڼ��ɣ�
��� �⣺��1�����Һ����ɫ��Һ��A�缫�����к�ɫ�Ĺ�̬�������ɣ�����A����ͭ���ӵõ��ӣ�����A����������A�ӵ��ǵ�Դ�ĸ�����B�缫����ɫ�������ɣ���һ����������ʧ���Ӳ�������������Ӧ��4OH--4e-=2H2O+O2�����ʴ�Ϊ������4OH--4e-=2H2O+O2����
��2��������Ӧ��2Cu2++4e-��2Cu��������Ӧ��4OH--4e-=2H2O+O2�������ʱ��Ӧ�������ӷ���ʽΪ��2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+4H++O2����ȡ��A�缫��ϴ�ӡ�����������缫����1.6g������������������ͭ��������1.6g����0.025mol������ͭ���ӵ�Ũ��=$\frac{0.025mol}{0.5L}$=0.05mol/L��ԭ��Һ�����ʵ���Ũ����0.05mol/L�����������ӵ����ʵ���Ϊ0.05mol������c��H+��=$\frac{0.05mol}{0.5L}$=0.1mol/L����pH=1���ʴ�Ϊ��0.05mol/L��1��
��3������Һ��һ���Ǻ�ͭ���ӵĿ����Ե�����Һ�����Կ���������ͭ����������ͭ���������Ȼ�ͭ����Ϊ�������������������������ӿ��Բ��ü����Ȼ�����Һ�ķ��������а�ɫ�������ɣ���ԭ��Һ����������������SO42-�������ỷ���µ���������ӣ����Լ������ͭ������ɫ�������ɣ����ڿ����б�Ϊ����ɫ����ԭ��Һ����������������NO3-��
�ʴ�Ϊ���ټ���ԭ��Һ�е������������������ӣ�ȡ�������Һ�������м���BaCl2��Һ�����а�ɫ�������ɣ���ԭ��Һ����������������SO42-���ڼ���ԭ��Һ�е������������������ӣ�ȡ�������Һ�������м���ͭ���ȣ���ͭ�ܽ⣬������ɫ�������ɣ����ڿ����б�Ϊ����ɫ����ԭ��Һ����������������NO3-��
���� ���⿼��ѧ�����صĹ���ԭ����ע��缫��Ӧʽ����д�Լ��缫��Ӧ�������غ�ļ����ǹؼ����ѶȲ���

| A�� | ��״���£�2.24Lú�ͣ��ٶ���ѧʽΪC8H18���к���0.8NA��̼ԭ�� | |
| B�� | ���³�ѹ�£�O2��O3�Ļ����16g�к���NA����ԭ�� | |
| C�� | 25��ʱ��1L 0.1mol•L-1������������Һ�к���NA��OH- | |
| D�� | 0.5mol CH4���0.5NA������ |
| A�� | ��Һ�е���Ҫ���ӽ�ΪSO${\;}_{3}^{2-}$��Fe2+��NH${\;}_{4}^{+}$ | |
| B�� | Cl-һ�����ڣ���c��Cl-��=0.2 mol��L-l | |
| C�� | ��ԭ��Һ�м������ᣬ�������������� | |
| D�� | CO${\;}_{3}^{2-}$��Al2+һ�������ڣ�K+���ܴ��� |
| ��ʼŨ�� | �� | �� | �� |
| c��H2/mol•L-1 | 0.010 | 0.020 | 0.020 |
| c��I2��/mol•L-1 | 0.010 | 0.010 | 0.020 |
| A�� | ƽ��ʱ���кͱ���H2��ת������ͬ | |
| B�� | ƽ��ʱ����I2��ת����С��40% | |
| C�� | ƽ��ʱ����c��HI���ȼ��е�2���� | |
| D�� | ��Ӧƽ��ʱ������������ɫ�������������ɫ��dz |
| A�� | �����Ʊ�����ˮ�� | |
| B�� | ��ˮ���ڴ��������Լ�ƿ�� | |
| C�� | ��������������ɫ���ƿ�У������������� | |
| D�� | ����������Һ������ĥ�ڲ��������Լ�ƿ�� |
| A�� | ������Ʒ���ڲ�ʹ��Ҳ���ϻ� | |
| B�� | ����ͨ��������Ļ���ʵ�����̼���ƺ�̼������ | |
| C�� | �������ƿ����������������ԺͶ�������Ӧ�����������ƺ����� | |
| D�� | ������ Na2C03��С�մ��� NaHC03����ˮ��Һ���ʼ��� |
| A�� | SO3+H2O�TH2SO4 | B�� | 2Na+2H2O�T2NaOH+H2�� | ||
| C�� | 2H2+O2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O | D�� | 2F2+2H2O�T4HF+O2 |
| A�� | ����ϡ��������Ͳ��ȡŨ����ʱ�����ӿ̶��� | |
| B�� | ����Ͳ��ȡ�����Ũ���ᵹ���ձ�������ˮϴ��Ͳ 2��3 �Σ�ϴҺ�����ձ��� | |
| C�� | ���� 11.7 g NaCl ���� 0.2 mol/L NaCl ��Һʱ��������������� | |
| D�� | ����ʱ���ӿ̶��� |