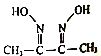��Ŀ����
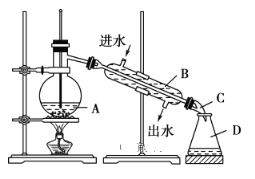
����Ŀ��ij������������Ա����������ȡ����������Ʒ1.00mL����ƿ�У�������������ˮϡ�ͣ�����ϡ�����ữ����μ���0.10 mol��L��1��KMnO4��Һ������Ӧ��ȫʱ������KMnO4��Һ25.00 mL����ط�Ӧ�Ļ�ѧ���ij�ʽΪ��2KMnO4��5H2O2��3H2SO4=K2SO4+2MnSO4��5O2����8H2O
(1)��֪�������ⲻ�ȶ�����д���������ⷢ���ֽⷴӦ�Ļ�ѧ����ʽ������˫���ű������ת�Ƶķ������Ŀ_____________________________________________________��
(2)ͨ������ȷ���ù���������Ʒ�����ʵ���Ũ��(д���������)______________��
���𰸡�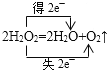 n(KMnO4)=0.10mol��L��1��25.00��10��3L=2.5��10��3mol
n(KMnO4)=0.10mol��L��1��25.00��10��3L=2.5��10��3mol
2 KMnO4 �� 5H2O2
2 5
2.5��10��3mol n(H2O2)
n(H2O2)=6.25��10��3mol
c(H2O2)= 6.25��10��3mol��0.001L=6.25mol/L
��������
���ݻ��ϼ۵ı仯�����Է���������ԭ��Ӧ�е���ת�Ƶķ������Ŀ���������ŷ���ʾ�����û�ѧ����ʽ�ɽ����й����ʵ����ļ��㡣
(1)�������ⲻ�ȶ������ܷ����ֽⷴӦ2H2O2=2H2O+O2������Ӧ����4����1����ԭ�ӣ�����2��ʧ2e-���0�ۣ���2����2e-��Ϊ��2������˫���ű������ת�Ƶķ������Ŀ

(2)�ɻ�ѧ����ʽ�ã� 2KMnO4 ~ 5H2O2
2 5
0.10 mol��L��1��25.00��10-3L c(H2O2)��1.00��10-3L
���c(H2O2)��6.25mol/L