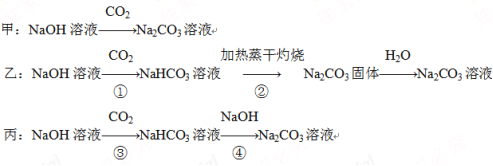
��Ŀ����
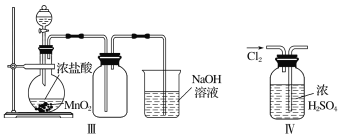
����Ŀ��Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�з����ķ�Ӧ��ijУ��ѧ��ȤС��ͬѧ�������ͼ��ʾ��ʵ��װ�á�
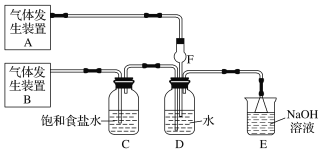
(1)�û�ѧ��ȤС���ͬѧΪ��ȡCl2��SO2���壬�ֲ���Na2SO3��70%������Ϊԭ����ȡSO2������MnO2��Ũ����(12 mol��L1)Ϊԭ����ȡCl2���ڴ�ʵ���У�F������������________������װ��BӦѡ����������װ���е�________(�����)��
(2)Dװ������Ҫ��Ӧ�����ӷ���ʽΪ_________________________________��
(3)Ϊ��֤ͨ��Dװ���е�������Cl2��������SO2��������ȤС���ͬѧ���������Լ���
���Ȼ�����Һ ���Ȼ�������Һ �����軯����Һ �����Ը��������Һ
��Cl2������ȡ����D����Һ�μ���ʢ��________(ѡ��һ�����)�Լ����Թ��ڣ��ټ���________(ѡ��һ�����)�Լ���������������______________________________________��
��SO2������ȡ����D����Һ�μ���ʢ��________(ѡ��һ�����)�Լ����Թ��ڣ�������������_______________________________________________________________��
���𰸡�(1)������ ��
(2)Cl2+SO2+2H2O===4H++![]() +2Cl
+2Cl
(3)�� �� ��Һ�ʺ�ɫ �� �Ϻ�ɫ��Ϊ��ɫ
��������(1)��ʵ��װ�ÿ�֪�����巢��װ��B�����������ñ���ʳ��ˮ���г��Ӿ�������֪Bװ��Ϊ��ȡCl2װ�ã���Aװ��Ϊ��ȡSO2��װ�ã���SO2������ˮ����F����������Ϊ����������ȡCl2�����Լ�ΪMnO2��Ũ���ᣬ���ڹ̡�Һ��ϼ�����ȡ���壬��Ӧѡ��װ��Ϊ����װ��B��
(2)��Cl2��SO2ͬʱͨ��ˮ��ʱ��Cl2��SO2��������H2SO4��Cl2����ԭΪHCl��
(3)��Cl2����������D����Һ�г���H2SO4��HCl�⣬������ʣ��Cl2��HClO������ǿ�����ԣ��ɽ�Fe2+����ΪFe3+���ʿ�ѡ���ڢ����м��飻��SO2����������D����Һ�лẬ��SO2��H2SO3��SO2���л�ԭ�ԣ��ʿ�ѡ�������м��顣