��Ŀ����
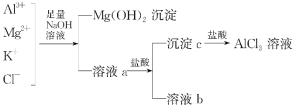
����Ŀ����ͼ��ʾΪһ����AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ����������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ�
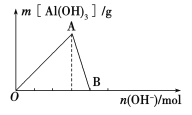
��ش��������⣺
(1)A��ʱ�Ѳμӷ�Ӧ��AlCl3��NaOH�����ʵ���֮��Ϊ________��
(2)AB����������ʾ�ķ�Ӧ�����ӷ���ʽΪ_________________________��
(3)��B�����ɵ���Һ��ͨ�������̼���ɹ۲쵽��������_______________________��
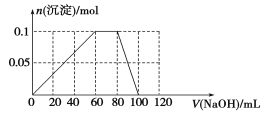
(4)����0.1 mol NH4Al(SO4)2����Һ����μ���5 mol��L1 NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����д̼�����ζ�������ݳ�������ɫ�������ٲ�������ʧ��������ͼ�л������ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵʾ��ͼ��
���𰸡�(1)13
(2)Al(OH)3+OH===![]() +2H2O
+2H2O
(3)���ɰ�ɫ����
(4)
��������(1)A������ﵽ���OA�η������ӷ�Ӧ����ʽΪ��Al3++3OH===Al(OH)3�����������ʵ���֮��Ϊ1��3��
(2)��������������������������AB�η������ӷ�Ӧ����ʽΪ��Al(OH)3+OH===![]() +2H2O��
+2H2O��
(3)B������ΪNaAlO2��ͨ�������̼������Ӧ��2![]() +CO2+3H2O===2Al(OH)3��+
+CO2+3H2O===2Al(OH)3��+![]() ������а�ɫ����������
������а�ɫ����������
(4)Al3+��OH������ǿ��![]() ������ȷ���Al3++OH===Al(OH)3��������������ʱ������NaOH�����Ϊ60 mL��Ȼ����
������ȷ���Al3++OH===Al(OH)3��������������ʱ������NaOH�����Ϊ60 mL��Ȼ����![]() +OH===NH3��H2O����ʱ�������������䣬�˽����ĵ�NaOH�����Ϊ20 mL�����������Al(OH)3+OH===
+OH===NH3��H2O����ʱ�������������䣬�˽����ĵ�NaOH�����Ϊ20 mL�����������Al(OH)3+OH===![]() +2H2O����ʱ���ĵ�NaOH�����Ϊ20 mL�����ͼ�������ʾ��
+2H2O����ʱ���ĵ�NaOH�����Ϊ20 mL�����ͼ�������ʾ��