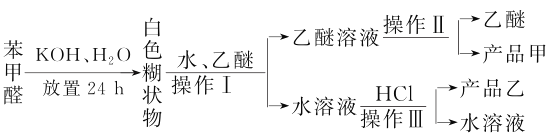
��Ŀ����
����Ŀ��ij�����ĩ������Na2O��Na2O2��Na2CO3��NaHCO3��NaCl�е�һ�ֻ�����ɡ����÷�ĩ�����������ᷴӦ������X�ݳ���Xͨ��������NaOH��Һ�������С(ͬ�¡�ͬѹ�²ⶨ)��ʣ�����塣����ԭ����Ϸ�ĩ�ڿ������þƾ��Ƽ��ȣ�Ҳ������ų�����ʣ��������������ԭ��Ϸ�ĩ�������������ж���ȷ����( )
�ٷ�ĩ��һ����Na2O��Na2O2��NaHCO3
�ڷ�ĩ��һ��������Na2CO3��NaCl
�۷�ĩ��һ��������Na2O��NaCl
�����϶���ĩ���Ƿ���Na2CO3��NaCl
A���٢� B���ڢ� C���ۢ� D���٢�
���𰸡�D
�������������ᷴӦ������������ʿ���ΪNa2O2(����O2)��NaHCO3��Na2CO3(����CO2)������Xͨ��NaOH��Һ�������С��ʣ�����壬˵��X��O2��CO2��ɣ�ԭ��ĩ��һ����Na2O2��Na2CO3��NaHCO3������һ�֡���ԭ��ĩ���ȣ�������ų���˵���������һ����NaHCO3����NaHCO3���ȷֽ��ʹ�����ĩ�������٣���ʵ����ʣ����������ȴ�����ˣ�ԭ��ֻ���Ƿ����˷�Ӧ��2Na2O��O2![]() 2Na2O2�����Ϸ������������һ����Na2O��Na2O2��NaHCO3����ȷ����������Ƿ���Na2CO3��NaCl��
2Na2O2�����Ϸ������������һ����Na2O��Na2O2��NaHCO3����ȷ����������Ƿ���Na2CO3��NaCl��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�