��Ŀ����
��2012?�ൺ���ع�ʵ���̽�������뽫����ʵ�鱨���еĿ�ȱ������д������
��1��̽��Ӱ�������ߵ͵����أ�
��2��̽����ķ��䶨�ɣ�
��3��̽��������ۻ����ɣ�
��1��̽��Ӱ�������ߵ͵����أ�
| ���� | �ڸ���ʵ���У�Ҫע��ʹ�ֳ������ķ��� ��ͬ ��ͬ �� |
| ���� | ��ʵ��ó���������Խ�죬������Խ �� �� �� |
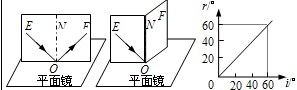
| ���̽��� | ��ͼʵ��˵�����ڹ�ķ����У�������ߡ�������ߺͷ����� ͬһƽ���� ͬһƽ���� ����ͼ��ó����ڹ�ķ����У����� ���� ���� ����� |
 |
| ���� | �о���ķ���ʱ����һ���⿴��һ�����ߣ��������õĿ�ѧ������ ģ�� ģ�� ���� | |
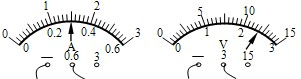
| ���� | ����ʵ��ͼ���֪���ù����� �� �� �壬ab�����ʴ�����Һ���� ��Һ���� ״̬�� | ||||||||||||
| ���� | ͨ�����ʵ�飬�õ����ɣ����������
|
 | |||||||||||
��������1�������������ߵ��ɷ������Ŀ��������ģ���������Խ�죬����Խ�ߣ���Խ��������Խ�ͣ�ע��ȷ���������ķ�����ͬ��
��2���ڹ�ķ��ķ��������У�������ߡ�������ߡ�������ͬһƽ���ڣ�����ǵ�������ǣ����ʵ���������ģ�͵ķ�������̽���ģ�
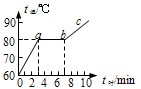
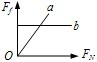
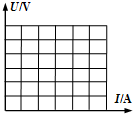
��3����ͼ���б������Ǿ�����Ҫ�����������Ƿ���һ�����۵㣬����һ��ʱ�������������ȣ����¶Ȳ����ߣ�����ʱ�������������ۻ��Ĺ��̣�
��2���ڹ�ķ��ķ��������У�������ߡ�������ߡ�������ͬһƽ���ڣ�����ǵ�������ǣ����ʵ���������ģ�͵ķ�������̽���ģ�
��3����ͼ���б������Ǿ�����Ҫ�����������Ƿ���һ�����۵㣬����һ��ʱ�������������ȣ����¶Ȳ����ߣ�����ʱ�������������ۻ��Ĺ��̣�
����⣺��1��Ϊ��̽��Ӱ�������ߵ͵����أ�����Ʒ�����IJ��Ϻ��ķ�����ͬ�������ֳ�������ͬʱ���ĸֳ�Խ��������Խ�ߣ�
��2����ֽ����NO����ۣ�E����F�治��ͬһƽ���ڣ��۲�F������û�з���⣬�ɼ���������ߡ�������ߡ������Ƿ���ͬһƽ���ϣ���ͼ���֪�����ʼ�յ�������ǣ�
�о���ķ���ʱ����һ���⿴��һ�����ߣ��������õĿ�ѧ������ģ�ͷ���
��3����ͼ��֪��ab��ʱ�����������ȣ����¶Ȳ������ߣ�˵����ʱ���ʴﵽ���۵㣬�����ۻ�����������������ھ��壬���Ҵ�ʱ���ʴ��ڹ�Һ�����״̬�����۾��廹�ǷǾ����ۻ�ʱ����Ҫ���ȣ�
�ʴ�Ϊ����1����ͬ���ߣ���2��ͬһƽ���ڣ����ڣ�ģ�ͣ���3��������Һ���棻���ȣ�
��2����ֽ����NO����ۣ�E����F�治��ͬһƽ���ڣ��۲�F������û�з���⣬�ɼ���������ߡ�������ߡ������Ƿ���ͬһƽ���ϣ���ͼ���֪�����ʼ�յ�������ǣ�
�о���ķ���ʱ����һ���⿴��һ�����ߣ��������õĿ�ѧ������ģ�ͷ���
��3����ͼ��֪��ab��ʱ�����������ȣ����¶Ȳ������ߣ�˵����ʱ���ʴﵽ���۵㣬�����ۻ�����������������ھ��壬���Ҵ�ʱ���ʴ��ڹ�Һ�����״̬�����۾��廹�ǷǾ����ۻ�ʱ����Ҫ���ȣ�
�ʴ�Ϊ����1����ͬ���ߣ���2��ͬһƽ���ڣ����ڣ�ģ�ͣ���3��������Һ���棻���ȣ�
������������һ��ʵ��̽���⣬Ҫע��۲�ʵ������Ȼ�����������ֵ�ԭ�����ó����ۣ�ͬʱע�ⲻͬʵ��Ӧ�õ�̽������������������Ҫ��Ƚ�ǿ����һ�����Ѷȣ���һ���е��⣮

��ϰ��ϵ�д�
�����Ŀ


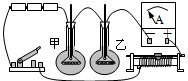
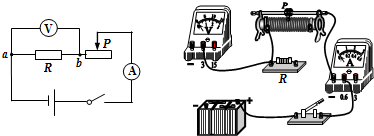
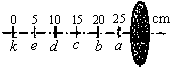 ��2007?�ൺ���ع�ʵά��̽����
��2007?�ൺ���ع�ʵά��̽����