��Ŀ����
��ͼ��ʾ��С����̽��Ӱ�컬��Ħ������С�����ء���ʵ�飮ͭ���ľ��Ĵ�С����״��ȫ��ͬ����ʵ��ǰ��С����������¼��ֲ��룺
����һ������Ħ�����Ĵ�С��ѹ���Ĵ�С�й�
�����������Ħ�����Ĵ�С�������Ӵ���Ĵֲڳ̶��й�
������������Ħ�����Ĵ�С�������ĽӴ������С�й�
��1��ʵ��ʱ���ɲ���������������ˮƽ������
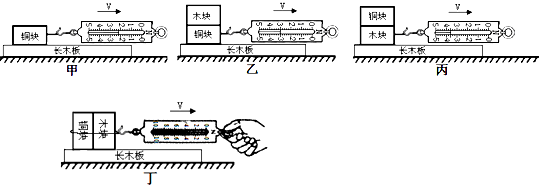
��2���Ƚϼס�����ͼ���ɵõ��Ľ�����
��3��ͼ�ҡ�����ͭ���ľ�����һ���Ŀ����ʹ
��4��Ҫ��̽������Ħ������С��Ӵ������С�Ƿ��йء���С����ľ���ͭ����Ϊһ���������ŷ����ڳ�ľ���ϣ��綡ͼ���������Ħ�����Ĵ�С�����ұȽϣ�����Ϊ����̽�������д��ڵ���Ҫȱ���ǣ�
��5����ȫ�ཻ�������У�С��Ϊ̽�������������������·�����
���õ��ɲ����Ʋ��ͭ���ľ���������С�ֱ�ΪG1��G2��
����ͼA��B��ʾ����ͭ���ľ�鶼ƽ���ڳ�ľ���ϲ������Ħ������С�ֱ�Ϊf1��f2��
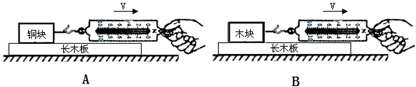
�۱Ƚ�
��
�Ĵ�С��ϵ����ý��ۣ�����ʾ����ʱ����ľ���ѹ����С��Ϊ������С����
��ʦ�϶���С���ķ�������Ϊ��
����·����ʱ��ı�ֵ����λʱ��������ͨ����·�̣���ӳ�������˶��Ŀ�����ͬ������������Ħ������ѹ���ı�ֵ��
��������ѧ�������ñ�ֵ�����������������
��6����ȫ�ཻ�������У�ͬ���С����ʵ���л����֣������廹û�б�����������£����ɲ�������Ȼ��ʾ������ʾ��������ֱ����������Ϊֹ����С���۲쵽�������������һ���м�ֵ�ҿ�̽��������
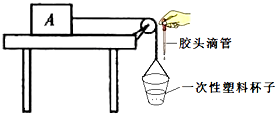
��7���κ༶����С���ͬѧ����Ƴ���ͼ��ʾ��װ�ò⻬��Ħ�����������ϵĻ���Aͨ�������ƹ����֣�������һ������һֻһ�������ϱ��ӣ�����ʱ�������ϱ��м�����ˮ�����������ˮʱ�ɸ��ý�ͷ�ιܣ���ʹ����A������ֱ���˶�����һ���Ա��е�ˮȫ�����������У�����������ˮ�����250ml����A�ܵ���Ħ����f=
����һ������Ħ�����Ĵ�С��ѹ���Ĵ�С�й�
�����������Ħ�����Ĵ�С�������Ӵ���Ĵֲڳ̶��й�
������������Ħ�����Ĵ�С�������ĽӴ������С�й�
��1��ʵ��ʱ���ɲ���������������ˮƽ������
����ֱ��
����ֱ��
�˶�����ʱ���ɲ����Ƶ�ʾ����Ϊ�������ܻ���Ħ�����Ĵ�С���������������ǣ�����ƽ��
����ƽ��
��
��2���Ƚϼס�����ͼ���ɵõ��Ľ�����
�Ӵ���ֲڳ̶�һ����ѹ��Խ����Ħ����Խ��
�Ӵ���ֲڳ̶�һ����ѹ��Խ����Ħ����Խ��
����3��ͼ�ҡ�����ͭ���ľ�����һ���Ŀ����ʹ
ѹ��
ѹ��
��ͬ���Ƚ��ҡ�����ͼ�ɵó�����Ħ�����Ĵ�С���Ӵ���ֲڳ̶�
�Ӵ���ֲڳ̶�
�йأ���4��Ҫ��̽������Ħ������С��Ӵ������С�Ƿ��йء���С����ľ���ͭ����Ϊһ���������ŷ����ڳ�ľ���ϣ��綡ͼ���������Ħ�����Ĵ�С�����ұȽϣ�����Ϊ����̽�������д��ڵ���Ҫȱ���ǣ�
û�п��ƽӴ���Ĵֲڳ̶���ͬ
û�п��ƽӴ���Ĵֲڳ̶���ͬ
����5����ȫ�ཻ�������У�С��Ϊ̽�������������������·�����
���õ��ɲ����Ʋ��ͭ���ľ���������С�ֱ�ΪG1��G2��
����ͼA��B��ʾ����ͭ���ľ�鶼ƽ���ڳ�ľ���ϲ������Ħ������С�ֱ�Ϊf1��f2��

�۱Ƚ�
| f1 |
| G1 |
| f2 |
| G2 |
��ʦ�϶���С���ķ�������Ϊ��
����·����ʱ��ı�ֵ����λʱ��������ͨ����·�̣���ӳ�������˶��Ŀ�����ͬ������������Ħ������ѹ���ı�ֵ��
��λѹ��
��λѹ��
�µĻ���Ħ��������ӳ���Ӵ���Ĵֲڳ̶�
�Ӵ���Ĵֲڳ̶�
����������ѧ�������ñ�ֵ�����������������
�ܶȣ�ѹǿ��
�ܶȣ�ѹǿ��
����6����ȫ�ཻ�������У�ͬ���С����ʵ���л����֣������廹û�б�����������£����ɲ�������Ȼ��ʾ������ʾ��������ֱ����������Ϊֹ����С���۲쵽�������������һ���м�ֵ�ҿ�̽��������
��Ħ�����Ĵ�С�Ƿ��������Ĵ�С�й�
��Ħ�����Ĵ�С�Ƿ��������Ĵ�С�й�
����7���κ༶����С���ͬѧ����Ƴ���ͼ��ʾ��װ�ò⻬��Ħ�����������ϵĻ���Aͨ�������ƹ����֣�������һ������һֻһ�������ϱ��ӣ�����ʱ�������ϱ��м�����ˮ�����������ˮʱ�ɸ��ý�ͷ�ιܣ���ʹ����A������ֱ���˶�����һ���Ա��е�ˮȫ�����������У�����������ˮ�����250ml����A�ܵ���Ħ����f=
2.5
2.5
N��ʵ����ѡ��һ�������ϱ��ӵ�ԭ���ǣ�����С
����С
����α��е�װ�ñȽϺ������ָ�����ø�װ�õ��ŵ������ڽ�ȷ�ؿ�������������ֱ���˶�������������˶��еĵ��ɲ����Ʒ������
���ڽ�ȷ�ؿ�������������ֱ���˶�������������˶��еĵ��ɲ����Ʒ������
��
��������1���Ӷ���ƽ��ĽǶ��������ش�
��2�����ݿ��Ʊ���������ȷ�����Ͳ��������ٽ�һ�����ݱ仯��ϵ�ó����ۣ�
��3������齻��λ�õ��ŵ�Ŀ����Ϊ�˿���ѹ����ͬ���Ӵ���Ĵֲڳ̶Ȳ�ͬ���ݴ������з�����
��4����Ҫ̽��Ħ������С��Ӵ�����Ĺ�ϵ��Ӧ����ѹ���ͽӴ���Ĵֲڳ̶Ⱦ���ͬ��
��5��ͨ���Ա�ֵ���巨�����⣬����ʵ��������ƻ��ڵ����壻
��6����������������˶������ƣ���ȴ��û���˶�ʱ��������Ħ��Ϊ��Ħ�����ݴ˿�����м�ֵ�����⣻
��7��ͼ�б���ˮ�������͵��ڶ�����A���������ݴ˿ɽ��м��㣮ͬʱ����ʵ���н������ֽ���������������ֱ���˶���ˮ������Ҳ��ͨ�������ȼ�Ӳ�����
��2�����ݿ��Ʊ���������ȷ�����Ͳ��������ٽ�һ�����ݱ仯��ϵ�ó����ۣ�
��3������齻��λ�õ��ŵ�Ŀ����Ϊ�˿���ѹ����ͬ���Ӵ���Ĵֲڳ̶Ȳ�ͬ���ݴ������з�����
��4����Ҫ̽��Ħ������С��Ӵ�����Ĺ�ϵ��Ӧ����ѹ���ͽӴ���Ĵֲڳ̶Ⱦ���ͬ��
��5��ͨ���Ա�ֵ���巨�����⣬����ʵ��������ƻ��ڵ����壻
��6����������������˶������ƣ���ȴ��û���˶�ʱ��������Ħ��Ϊ��Ħ�����ݴ˿�����м�ֵ�����⣻
��7��ͼ�б���ˮ�������͵��ڶ�����A���������ݴ˿ɽ��м��㣮ͬʱ����ʵ���н������ֽ���������������ֱ���˶���ˮ������Ҳ��ͨ�������ȼ�Ӳ�����
����⣺��1��ֻ�е�����ƽ��ʱ������Ħ��������ȣ�����Ҫ���ʱҪ������ֱ���˶���
��2�����ݿ��Ʊ�������������ͼ�нӴ���Ĵֲڳ̶���ͬ��ѹ����ͬ��Ħ������ͬ����˿ɵó����ۣ��Ӵ���ֲڳ̶�һ����ѹ��Խ����Ħ����Խ��
��3������齻��λ�õ��ŵ�Ŀ����Ϊ�˿���ѹ����ͬ���Ƚ��ҡ�����ͼ�ɵó�����Ħ�����Ĵ�С��Ӵ���Ĵֲڳ̶��йأ�
��4����ͼ�У�������ţ���Ȼѹ����ͬ����û�п��ƽӴ���Ĵֲڳ̶���ͬ�����Խ���û��˵������
��5������С������Ƶķ�����֪������һ��ͨ����ֵ�������еıȽϣ���ˣ�������֪������Ħ������ѹ���ı�ֵ����λѹ���µĻ���Ħ��������ӳ�˽Ӵ���Ĵֲڳ̶ȣ��������ñ�ֵ��������������������ܶȡ�ѹǿ�����ʵȣ�
��6����������������˶�������ʱ��������Ħ������Ϊ��Ħ���������ǿ����о���Ħ�����Ĵ�С��������С�Ĺ�ϵ��
��7��ͼ�б���ˮ�������͵��ڶ�����A���������͵��������������Ħ�������ڲ��Ʊ���ʱ��f=F=G=mg=��Vg=1.0��103kg/m3��250��10-6m3��10N/kg=2.5N��
ʵ����ֽ��������С���ɺ��Բ��ƣ���������ʱ�����㣻
ͬʱ�����û��ֿɽϺõؿ�������������ֱ���˶���Ҳ����ʡȥʹ�õ��ɲ����ƣ��˷���������ȱ�㣮
�ʴ�Ϊ��
��1������ֱ���˶��� ����ƽ�⣻
��2���Ӵ���ֲڳ̶�һ����ѹ��Խ����Ħ����Խ��
��3��ѹ���� �Ӵ���Ĵֲڳ̶��йأ�
��4��û�п��ƽӴ���Ĵֲڳ̶���ͬ��
��5����λѹ���� �Ӵ���Ĵֲڳ̶ȣ� �ܶȣ�
��6����Ħ�����Ĵ�С�Ƿ��������Ĵ�С�йأ�
��7��2.5�� ����С�����ܶ�С�������ɺ��Բ��Ƶȱ��������ԣ��� ���ڽ�ȷ�ؿ�������������ֱ���˶�������������˶��еĵ��ɲ����Ʒ��������
��2�����ݿ��Ʊ�������������ͼ�нӴ���Ĵֲڳ̶���ͬ��ѹ����ͬ��Ħ������ͬ����˿ɵó����ۣ��Ӵ���ֲڳ̶�һ����ѹ��Խ����Ħ����Խ��
��3������齻��λ�õ��ŵ�Ŀ����Ϊ�˿���ѹ����ͬ���Ƚ��ҡ�����ͼ�ɵó�����Ħ�����Ĵ�С��Ӵ���Ĵֲڳ̶��йأ�
��4����ͼ�У�������ţ���Ȼѹ����ͬ����û�п��ƽӴ���Ĵֲڳ̶���ͬ�����Խ���û��˵������
��5������С������Ƶķ�����֪������һ��ͨ����ֵ�������еıȽϣ���ˣ�������֪������Ħ������ѹ���ı�ֵ����λѹ���µĻ���Ħ��������ӳ�˽Ӵ���Ĵֲڳ̶ȣ��������ñ�ֵ��������������������ܶȡ�ѹǿ�����ʵȣ�
��6����������������˶�������ʱ��������Ħ������Ϊ��Ħ���������ǿ����о���Ħ�����Ĵ�С��������С�Ĺ�ϵ��
��7��ͼ�б���ˮ�������͵��ڶ�����A���������͵��������������Ħ�������ڲ��Ʊ���ʱ��f=F=G=mg=��Vg=1.0��103kg/m3��250��10-6m3��10N/kg=2.5N��
ʵ����ֽ��������С���ɺ��Բ��ƣ���������ʱ�����㣻
ͬʱ�����û��ֿɽϺõؿ�������������ֱ���˶���Ҳ����ʡȥʹ�õ��ɲ����ƣ��˷���������ȱ�㣮
�ʴ�Ϊ��
��1������ֱ���˶��� ����ƽ�⣻
��2���Ӵ���ֲڳ̶�һ����ѹ��Խ����Ħ����Խ��
��3��ѹ���� �Ӵ���Ĵֲڳ̶��йأ�
��4��û�п��ƽӴ���Ĵֲڳ̶���ͬ��
��5����λѹ���� �Ӵ���Ĵֲڳ̶ȣ� �ܶȣ�
��6����Ħ�����Ĵ�С�Ƿ��������Ĵ�С�йأ�
��7��2.5�� ����С�����ܶ�С�������ɺ��Բ��Ƶȱ��������ԣ��� ���ڽ�ȷ�ؿ�������������ֱ���˶�������������˶��еĵ��ɲ����Ʒ��������
�����������һ��ʵ�����֣������˶��֪ʶ�㣬�������Ʊ����������á�����ƽ������á���ֵ���巨�����⡢ʵ��ĸĽ�����Ƶȣ��������������һ�����Ѷȣ��ۺ��Խ�ǿ��

��ϰ��ϵ�д�
�����Ŀ
 ��2010?������һģ����ͼ��ʾ��С��̽��Ӱ�������ЧӦ��ʵ���·�������ͼ�����رպ�һ��ʱ�����������ƿú���е�Һ�涼û�иı䣬С���϶���·�г����˶�·���������õ�ѹ����ʼ����·λ�ã���ش��й�������·���ϵ����⣺��˵������Դ��ã���·ֻ��һ����·���ϣ���
��2010?������һģ����ͼ��ʾ��С��̽��Ӱ�������ЧӦ��ʵ���·�������ͼ�����رպ�һ��ʱ�����������ƿú���е�Һ�涼û�иı䣬С���϶���·�г����˶�·���������õ�ѹ����ʼ����·λ�ã���ش��й�������·���ϵ����⣺��˵������Դ��ã���·ֻ��һ����·���ϣ��� ��2013?������һģ����ͼ��ʾ��С��̽����������������ܴ�С������������ͱ�����ĸ߶��Ƿ��йء��Ļ����������ʵ����龰��
��2013?������һģ����ͼ��ʾ��С��̽����������������ܴ�С������������ͱ�����ĸ߶��Ƿ��йء��Ļ����������ʵ����龰��
 ��ͼ��ʾ��С����̽��������·�е�ѹ�Ĺ�ϵ����ʵ���·ͼ��
��ͼ��ʾ��С����̽��������·�е�ѹ�Ĺ�ϵ����ʵ���·ͼ��