��Ŀ����
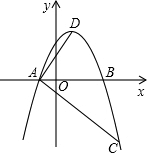
��ƽ��ֱ������ϵ�У������߹�ԭ��O������x�ύ����һ��A��A��O�Ҳࣩ������ΪB����˼��ͬѧ��һ�ѿ�3cm�ľ���ֱ�߶������߽������²�������1������OA=3cm����2������ֱ�ߵ�����������ߵĶԳƳ��غϣ�ʹ��ֱ�����¶˵��������ߵĶ����غ�ʱ����ͼ1���������������ֱ���ұߵĽ���C�Ŀ̶ȶ���Ϊ4.5cm��
��˼��ͬѧ��A�����������3��0����Ȼ�������������۳���������и��⣺
��1��д�������ߵĶԳ��
��2������������ߵĽ���ʽ��
��3��̽�������ߵĶԳ������Ƿ����ʹ��ACD�ܳ���С�ĵ�D��
��4��Ȼ���ֽ�ͼ�е�ֱ�ߣ��㹻������ˮƽ��������ƽ�Ƶ���A���ұߣ���ͼ2����ֱ�ߵ����߽�x���ڵ�H��G������������E��F��̽������EFGH�����S���߶�EF�ij����Ƿ���ں�����ϵ��
ͬѧ����������3����4�����۴��ڣ�����ﰬ˼��ͬѧһ����ɣ���������3����4�����۲����ڣ�������߰�˼��ͬѧ���۲����ڵ����ɣ�

�⣺��1���������߹�ԭ��O������x�ύ����һ��A��A��O�Ҳࣩ��OA=3��
��A��������3��0����
�������ߵĶԳ���Ϊֱ��x= ��
��
��2���������ߵĶԳ���Ϊֱ��x= ��
��
����������ߵĽ���ʽΪy=a��x- ��2+k��
��2+k��
�ඥ��B������Ϊ�� ��k����
��k����
��ͼ1���ߵ�C�ĺ�����Ϊ��ON= +3=
+3=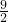 ����C��������y=a��x-
����C��������y=a��x- ��2+k�ϣ�
��2+k�ϣ�
���C��������Ϊa��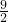 -
- ��2+k=9a+k��
��2+k=9a+k��
 ��MC=4.5��
��MC=4.5��
��9a+k-k=4.5��
��a= ��
��
��A�����꣨3��0������y= ��x-
��x- ��2+k��
��2+k��
�� ��3-
��3- ��2+k=0�����k=-
��2+k=0�����k=-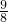 ��
��
�������ߵĽ���ʽΪy= ��x-
��x- ��2-
��2-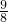 ����y=
����y= x2-
x2- x��
x��
��3�������ߵĶԳ����ϴ���ʹ��ACD�ܳ���С�ĵ�D���������£�
��ͼ1������OC���������ߵĶԳ����ڵ�D�����ACD���ܳ�=AC+AD+CD=AC+OD+CD=AC+OC��С��
��ֱ��OC�Ľ���ʽΪy=mx������C�����꣨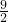 ��
��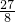 �����룬
�����룬
��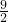 m=
m=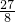 �����m=
�����m=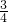 ��
��
��ֱ��OC�Ľ���ʽΪy=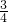 x��
x��
��x= ʱ��y=
ʱ��y=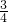 ��
�� =
=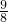 ��
��
������D�������� ��
��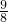 ����
����
��4������EFGH�����S���߶�EF�ij��ȴ��ں�����ϵ���������£�
��ͼ2�����E������Ϊa����E������Ϊ��a�� a2-
a2- a����H��������a��0����
a����H��������a��0����
��F������Ϊa+3��F������Ϊ��a+3�� ��a+3��2-
��a+3��2- ��a+3������G��������a+3��0����
��a+3������G��������a+3��0����
������EFGH�����S= ��EH+FG��•HG=
��EH+FG��•HG= [��
[�� a2-
a2- a��+
a��+ ��a+3��2-
��a+3��2- ��a+3��]��3=
��a+3��]��3= a2��
a2��
�֡� ��a+3��2-
��a+3��2- ��a+3��-��
��a+3��-�� a2-
a2- a��=3a��EF=
a��=3a��EF=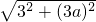 =3
=3 ��
��
��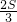 =
= -1��
-1��
��S=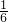 EF2-
EF2- ����S��EF���ȵĶ��κ�����
����S��EF���ȵĶ��κ�����
��������1���������߹�ԭ��O��A�㣨3��0�������������ߵĶԳ��ԣ����е����깫ʽ��������������ߵĶԳ���Ϊֱ��x=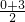 ����x=
����x= ��
��
��2�����������ߵĶԳ���Ϊֱ��x= ���������ߵĽ���ʽΪ����ʽy=a��x-
���������ߵĽ���ʽΪ����ʽy=a��x- ��2+k����B������Ϊ��
��2+k����B������Ϊ�� ��k�����ٽ�x=
��k�����ٽ�x=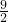 ���룬�����C��������Ϊ9a+k������MC=4.5�����a=
���룬�����C��������Ϊ9a+k������MC=4.5�����a= ��Ȼ��A�����꣨3��0������y=
��Ȼ��A�����꣨3��0������y= ��x-
��x- ��2+k�����k=-
��2+k�����k=-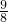 ���õ������ߵĽ���ʽΪy=
���õ������ߵĽ���ʽΪy= ��x-
��x- ��2-
��2-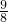 ����y=
����y= x2-
x2- x��
x��
��3������O��A������������ߵĶԳ���Գƣ���������OC���������ߵĶԳ����ڵ�D�����ACD���ܳ���С�������ô���ϵ�������ֱ��OC�Ľ���ʽ���ٽ�x= ���룬���y��ֵ�����ɵõ�D�����ꣻ
���룬���y��ֵ�����ɵõ�D�����ꣻ
��4�����ú�a�Ĵ���ʽ�ֱ��ʾE��H��F��G�ĵ�����꣬�õ�EH��FG�ij��ȣ��ٸ������ε������ʽ���S= a2������������֮��ľ��빫ʽ���EF=3
a2������������֮��ľ��빫ʽ���EF=3 ����
����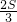 =
= -1��������ó�S=
-1��������ó�S=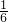 EF2-
EF2- ����S��EF���ȵĶ��κ�����
����S��EF���ȵĶ��κ�����
�����������Ƕ��κ������ۺ����ͣ������漰����֪ʶ�������ô���ϵ��������������������κ����Ľ���ʽ�����κ��������ʣ�ƽ�ơ���ԳƵ����ʣ����ε����������֮��ľ��빫ʽ���ۺ��Խ�ǿ���Ѷ����У����������ߵ��������ô���ϵ����������κ����Ľ���ʽ�ǽ���Ĺؼ���
��A��������3��0����
�������ߵĶԳ���Ϊֱ��x=
 ��
����2���������ߵĶԳ���Ϊֱ��x=
 ��
������������ߵĽ���ʽΪy=a��x-
 ��2+k��
��2+k���ඥ��B������Ϊ��
 ��k����
��k������ͼ1���ߵ�C�ĺ�����Ϊ��ON=
 +3=
+3=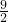 ����C��������y=a��x-
����C��������y=a��x- ��2+k�ϣ�
��2+k�ϣ����C��������Ϊa��
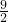 -
- ��2+k=9a+k��
��2+k=9a+k�� ��MC=4.5��
��MC=4.5����9a+k-k=4.5��
��a=
 ��
����A�����꣨3��0������y=
 ��x-
��x- ��2+k��
��2+k����
 ��3-
��3- ��2+k=0�����k=-
��2+k=0�����k=-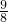 ��
���������ߵĽ���ʽΪy=
 ��x-
��x- ��2-
��2-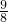 ����y=
����y= x2-
x2- x��
x����3�������ߵĶԳ����ϴ���ʹ��ACD�ܳ���С�ĵ�D���������£�
��ͼ1������OC���������ߵĶԳ����ڵ�D�����ACD���ܳ�=AC+AD+CD=AC+OD+CD=AC+OC��С��
��ֱ��OC�Ľ���ʽΪy=mx������C�����꣨
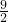 ��
��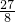 �����룬
�����룬��
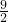 m=
m=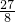 �����m=
�����m=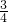 ��
����ֱ��OC�Ľ���ʽΪy=
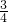 x��
x����x=
 ʱ��y=
ʱ��y=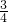 ��
�� =
=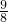 ��
��������D��������
 ��
��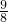 ����
������4������EFGH�����S���߶�EF�ij��ȴ��ں�����ϵ���������£�
��ͼ2�����E������Ϊa����E������Ϊ��a��
 a2-
a2- a����H��������a��0����
a����H������Ϊ��a��0������F������Ϊa+3��F������Ϊ��a+3��
 ��a+3��2-
��a+3��2- ��a+3������G��������a+3��0����
��a+3������G��������a+3��0����������EFGH�����S=
 ��EH+FG��•HG=
��EH+FG��•HG= [��
[�� a2-
a2- a��+
a��+ ��a+3��2-
��a+3��2- ��a+3��]��3=
��a+3��]��3= a2��
a2���֡�
 ��a+3��2-
��a+3��2- ��a+3��-��
��a+3��-�� a2-
a2- a��=3a��EF=
a��=3a��EF=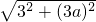 =3
=3 ��
����
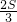 =
= -1��
-1����S=
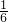 EF2-
EF2- ����S��EF���ȵĶ��κ�����
����S��EF���ȵĶ��κ�������������1���������߹�ԭ��O��A�㣨3��0�������������ߵĶԳ��ԣ����е����깫ʽ��������������ߵĶԳ���Ϊֱ��x=
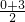 ����x=
����x= ��
����2�����������ߵĶԳ���Ϊֱ��x=
 ���������ߵĽ���ʽΪ����ʽy=a��x-
���������ߵĽ���ʽΪ����ʽy=a��x- ��2+k����B������Ϊ��
��2+k����B������Ϊ�� ��k�����ٽ�x=
��k�����ٽ�x=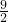 ���룬�����C��������Ϊ9a+k������MC=4.5�����a=
���룬�����C��������Ϊ9a+k������MC=4.5�����a= ��Ȼ��A�����꣨3��0������y=
��Ȼ��A�����꣨3��0������y= ��x-
��x- ��2+k�����k=-
��2+k�����k=-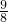 ���õ������ߵĽ���ʽΪy=
���õ������ߵĽ���ʽΪy= ��x-
��x- ��2-
��2-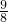 ����y=
����y= x2-
x2- x��
x����3������O��A������������ߵĶԳ���Գƣ���������OC���������ߵĶԳ����ڵ�D�����ACD���ܳ���С�������ô���ϵ�������ֱ��OC�Ľ���ʽ���ٽ�x=
 ���룬���y��ֵ�����ɵõ�D�����ꣻ
���룬���y��ֵ�����ɵõ�D�����ꣻ��4�����ú�a�Ĵ���ʽ�ֱ��ʾE��H��F��G�ĵ�����꣬�õ�EH��FG�ij��ȣ��ٸ������ε������ʽ���S=
 a2������������֮��ľ��빫ʽ���EF=3
a2������������֮��ľ��빫ʽ���EF=3 ����
����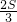 =
= -1��������ó�S=
-1��������ó�S=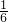 EF2-
EF2- ����S��EF���ȵĶ��κ�����
����S��EF���ȵĶ��κ����������������Ƕ��κ������ۺ����ͣ������漰����֪ʶ�������ô���ϵ��������������������κ����Ľ���ʽ�����κ��������ʣ�ƽ�ơ���ԳƵ����ʣ����ε����������֮��ľ��빫ʽ���ۺ��Խ�ǿ���Ѷ����У����������ߵ��������ô���ϵ����������κ����Ľ���ʽ�ǽ���Ĺؼ���

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ
 ����ԭ�㣮A��B����ĺ�����ֱ��Ƿ���x2-4x-12=0����������cos��DAB=
����ԭ�㣮A��B����ĺ�����ֱ��Ƿ���x2-4x-12=0����������cos��DAB= 18����ƽ��ֱ������ϵ�У���һ��ͼ��������ԭ��˳ʱ����ת�ĽǶ�Ϊ�ȣ�����ԭ��Ϊλ�����ģ����Ʊ�Ϊk�õ�һ���µ�ͼ�Σ����ǰ�������̼�Ϊ���ȣ�k���任�����磬��ͼ�еġ�ABC������ԭ��O˳ʱ����ת�ĽǶ�Ϊ90�㣬����ԭ��Ϊλ�����ģ����Ʊ�Ϊ2�õ�һ���µ�ͼ�Ρ�A1B1C1������������̼�Ϊ��90�㣬2���任��
18����ƽ��ֱ������ϵ�У���һ��ͼ��������ԭ��˳ʱ����ת�ĽǶ�Ϊ�ȣ�����ԭ��Ϊλ�����ģ����Ʊ�Ϊk�õ�һ���µ�ͼ�Σ����ǰ�������̼�Ϊ���ȣ�k���任�����磬��ͼ�еġ�ABC������ԭ��O˳ʱ����ת�ĽǶ�Ϊ90�㣬����ԭ��Ϊλ�����ģ����Ʊ�Ϊ2�õ�һ���µ�ͼ�Ρ�A1B1C1������������̼�Ϊ��90�㣬2���任��