��Ŀ����
�±��Ƕ�ij��У����ѧ�����������ֲ���ͬѧ�����ָ������쳣������ʾ�ޣ�����ʾ��������������ʾ���������ݱ������ش�
| ��Ʒ | �����Ŀ | ����ѧ������� | �����ο�ֵ��Χ | ||
| �� | �� | �� | |||
| ��� | ������ | �� | �� | �� | �� |
| ������ | ���� | �� | �� | �� | |
| ��ϸ�� | �� | �� | �� | �� | |
| ˮ | 95 | 96 | 95 | 95��97��g/100ml�� | |
| ���� | 1.4 | 1.2 | 1.5 | 0.9��1.6��g/100ml�� | |
| ���� | 1.9 | 1.8 | 2.0 | 1.8��2.0��g/100ml�� | |
| Ѫ�� | ��ϸ�� | 6.8 | 5.3 | 13.0 | 5.0��10.0(��109��/L) |
| ��ϸ�� | 4.8 | 3.0 | 5.1 | 3.5��5.5(��1012��/L | |
| Ѫ�쵰�� | 130 | 80 | 140 | 110��160(g/L) | |
��2������Һ�г� �ͺ�ϸ��������������в��䣬��ô���ܵIJ�λ������� ����
��3��ҽ��������Ѫ���к�ϸ����Ѫ�쵰����ֵ��ƫ�ͣ��ж������ܻ��� ����ƽʱ����ʳ���棬������Ľ����� ��
��4�����������ʱ�����巢�ף�����ɤ�ӡ����ۡ�Ѫ����ʾ ��˵����֢�δ��ڣ�����������ơ�
��1������ ���� ��2�������� ��С�� �����С�ұ�Ҳ�÷֣�
��3��ƶѪ ��ʳ�ú����͵����ʷḻ��ʳ� (����Ժ������ʷḻ��ʳ�
�������ĺ������ʷḻ��ʳ������ơ�������ḻ��ʳ���磺��ľ����������Ģ�����۲ˡ��²ˡ����ˡ���е���һ�ֵĶ��÷�) ��4����ϸ������ƫ��
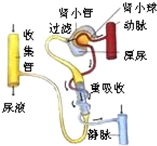
���������������1����Һ�ijɷ�Ϊˮ�����Σ����أ�������Һ�г��˴���ˮ���������κ����أ�
��2����Һ���γ��е�һ����С��Ĺ������ã���Ѫϸ���͵������⣬Ѫ����һ����ˮ�������ǣ����Σ����صȱ����˵���С�����γ�ԭ������������ԭ����û�е����ʺͺ�ϸ����һ������������С�����˲��䣬
��3����ϸ������Ѫ�쵰���ٻᵼ��ƶѪ�����ڳ���ĺ�ϸ������Ѫ�쵰�ף�����Ѫ�쵰����һ�ֺ����ĵ����ʣ�����ƶѪ����Ӧ����ʳ�ú����͵����ʷḻ��ʳ�
��4����ϸ��������ϸ�����������з����ͱ��������ã���������֢ʱ�����ڵİ�ϸ�����������ࡣ
���㣺���⿼�������Һ���γɺ�ѪҺ�ijɷ֣���Ҫ�����Լ�����Ľṹ���ܡ�
�����������Ϊ��ӱ������һ�����Ѷȣ�������ʱ����������������е����ݣ�Ȼ�����е���������صĻ���֪ʶ����ϵ���������ܽ���������Ŀ��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�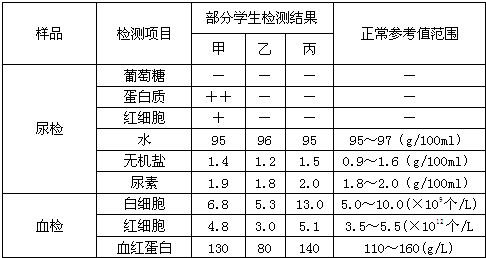
 ��2012?��ˮ��ģ�⣩��������ѧ��������ѧ֪ʶ������⣺
��2012?��ˮ��ģ�⣩��������ѧ��������ѧ֪ʶ������⣺ ��������ѧ��������ѧ֪ʶ������⣺
��������ѧ��������ѧ֪ʶ������⣺