��Ŀ����
����Ŀ��������ƿ��ɫ��Һ,�����������������������,��ѧʵ��С��ͬѧ�������ƿ�Լ����м���(��������,���ȿ��Ժ����ᷴӦ,Ҳ���Ժ�����������Һ��Ӧ,��������������Һ��Ӧ����ƫ������( NaAlO2)������)��
(1)����ͬѧ�������µ�ʵ��
ʵ�鲽�� | ʵ������ | ʵ����� |
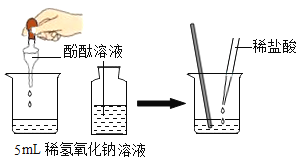
ȡһ֧�Թ�,����5ml��ɫҺ��,��������ɫ��ʯ���Լ� | ��Һ��________ɫ | ��ɫ��Һ��_______ |
ȡһ֧�Թ�,����5ml��ɫҺ��,��������ɫ��ʯ���Լ� | ��Һ��________ɫ | ��ɫ��Һ��NaOH |
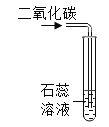
(2)����ͬѧ�õ����������е�ʵ�顣ȡһ֧�Թܼ���5��������ɫҺ��,�������������ɡ�Ȼ��ͬѧ�Ǿ�˵�������ᡣ�˷�Ӧ�Ļ�ѧ����ʽΪ:________________________��
(3)�����ͬѧ��Ϊ�˷����д�,����Ϊ����ͬѧ����������_______________����ѧ����ʽΪ______________��
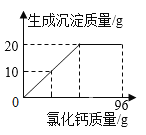
(4)����ͬѧ�����������ᷴӦ��ʵ��ʱ�ռ���������0.2��,��Ҫ����_______________��?������50�˵�������Һ,����Һ�����ʵ�����������____________?
���𰸡��� ϡ���� �� 2Al��6HCl=2AlCl3��3H2�� ��������������Һ��Ӧ����ƫ�����ƺ�������ͬ�������ݲ��� 2Al��2NaOH+2H2O=2NaAlO2��3H2�� 1.8 14.6%
��������
��1������ͬѧ�������µ�ʵ�飺
����һ��ȡһ֧�Թ�,����5ml��ɫҺ��,��������ɫ��ʯ���Լ�����Һ���ɫ��˵����ɫ��Һ�������ԣ������
ȡһ֧�Թ�,����5ml��ɫҺ��,��������ɫ��ʯ���Լ�����Һ����ɫ��˵����ɫ��Һ�Լ��ԣ���NaOH��
(2)����ͬѧ�õ����������е�ʵ�顣ȡһ֧�Թܼ���5��������ɫҺ��,�������������ɡ�Ȼ��ͬѧ�Ǿ�˵�������ᡣ����ϡ���ᷴӦ�����Ȼ����������Ļ�ѧ����ʽΪ��2Al��6HCl=2AlCl3��3H2����
(3)�����ͬѧ��Ϊ�˷����д�,����ͬѧ���������ǣ���������������Һ��Ӧ����ƫ�����ƺ�������ͬ�������ݲ���������������������Һ��Ӧ����ƫ�����ƺ������Ļ�ѧ����ʽΪ��2Al��2NaOH+2H2O=2NaAlO2��3H2����
(4)�⣺����Ҫ��������Ϊx���μӷ�Ӧ��ϡ������������Ϊy��
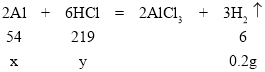
![]() x=1.8g
x=1.8g
![]() y=7.3g
y=7.3g
��Ҫ���۵�������1.8g��
ϡ�����������������=![]() ��
��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ�Ҳ�ǻ�ѧ����ᷢչ����������ľ��ס��ϳɰ�������ͼΪ:
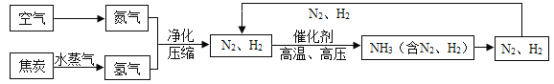
(1)��ȡ�����ķ�ӦΪ: 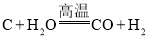 ����Ӧǰ�ϼ۷����仯��Ԫ����________________(��Ԫ�ط���)��
����Ӧǰ�ϼ۷����仯��Ԫ����________________(��Ԫ�ط���)��
(2)д��N2��H2��Ӧ����NH3�Ļ�ѧ����ʽ_______________________��
(3)����ͼ�н�N2��H2 ѭ��ʹ�ã�Ŀ����______________________��
(4)���е㲻ͬ��������뿪����������Һ�����뷨����:�����¶���- 183��Cʱ,�ɽ�������N2��O2���롣�����±������ʵķе��жϣ�Ҫ������NH3��N2��H2���뿪���������˵��¶�Ӧ�ÿ�����__________��
���� | H2 | N2 | O2 | NH3 |
�е� | -252��C | -195.8��C | -183��C | -33.35��C |
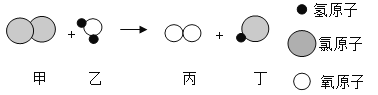
(5) ��һ�������£�A��B�ܷ�����ѧ��Ӧ����C��D,����ʾ��ͼ����
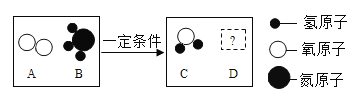
��DΪ��������������������壬��Ӧ��C��D�Ļ�ѧ������֮��Ϊ______________��
����Ŀ��������ѧϰ��ѧ���õķ�����
��1����ѧ��Ӧ����Щ��Ӧû������������Ҫ���ʵ��֤����ѧ��Ӧ�Ƿ�������ӷ�Ӧ���������仯�ĽǶȣ�������ʵ���е�A���______������B������C������Ϊһ�࣬�����Ǣ�____________��
|
|
|
A | B | C |
��2�����ʻ����ӵ���������������Ԫ�صĻ��ϼ۹�ϵ���У�
��ClO3-�����������ClO4-��������������������أ�K2O2���������أ�K2O���ȣ�
�ɴ��Ʋ�:MnO4-������Ϊ��__________���������ƵĻ�ѧʽΪ��___________��