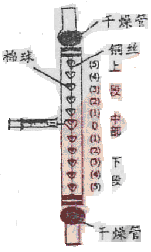
��Ŀ����
��2011���㽭���ݣ� 30�⣩Ϊ�˼��������Σ�����ҹ��з��ˡ���ˮ�����������ա����乤�����̴����ǣ�
I���Ӹߴ������µĺ�ˮ(pH=8.1-8.3)ϴ�Ѵ��¶���ȼú�����е�SO2, (SO2+H2O=H2SO3 H2SO3�����ᣩ����ˮ��Ϊ�����ԣ�
��.��ϴ��������ĺ�ˮ��������ֽӴ����������з�Ӧ��
4NaC1��O2ʮ2H2SO3 ==2Na2SO4��4HC1
III.�ٽ���������ֽӴ��ĺ�ˮ��������Ȼ��ˮ��ϵȴ�ʩ��ʹ����ָ��ӽ���Ȼ��ˮ�����ŷš�
(1)��������ͼ�л�������ˮ�����������ա�ȫ�����к�ˮ��pH���α仯�������ơ�
(2)��������������Ȼ��ˮ�Ӵ��������Ϊ�����ԣ���˵���ڴ˹����к�ˮ�е� ���Ӻ�H2SO3������������ӷ����˷�Ӧ��
(3)����Ҫ�ⶨ��ˮ��Na2SO4������ʵ����������ǣ�
ȡ�������⺣ˮ���ⶨ ������������ ��Һ��д��ѧʽ�����ټ���ϡ���ᡢ���ˡ�ϴ�ӡ���ɡ���������������
I���Ӹߴ������µĺ�ˮ(pH=8.1-8.3)ϴ�Ѵ��¶���ȼú�����е�SO2, (SO2+H2O=H2SO3 H2SO3�����ᣩ����ˮ��Ϊ�����ԣ�
��.��ϴ��������ĺ�ˮ��������ֽӴ����������з�Ӧ��
4NaC1��O2ʮ2H2SO3 ==2Na2SO4��4HC1
III.�ٽ���������ֽӴ��ĺ�ˮ��������Ȼ��ˮ��ϵȴ�ʩ��ʹ����ָ��ӽ���Ȼ��ˮ�����ŷš�
(1)��������ͼ�л�������ˮ�����������ա�ȫ�����к�ˮ��pH���α仯�������ơ�
(2)��������������Ȼ��ˮ�Ӵ��������Ϊ�����ԣ���˵���ڴ˹����к�ˮ�е� ���Ӻ�H2SO3������������ӷ����˷�Ӧ��
(3)����Ҫ�ⶨ��ˮ��Na2SO4������ʵ����������ǣ�
ȡ�������⺣ˮ���ⶨ ������������ ��Һ��д��ѧʽ�����ټ���ϡ���ᡢ���ˡ�ϴ�ӡ���ɡ���������������
(1)��ͼ

(2)����������OH-��
(3) ���⺣ˮ����������� BaCl2��Ba(N03)3��Ba(OH)2

(2)����������OH-��
(3) ���⺣ˮ����������� BaCl2��Ba(N03)3��Ba(OH)2
��������1�������������PHֵ�Ĺ�ϵ���DZ��⣻��2�����ݴӸߴ������µĺ�ˮ��pH=8.1-8.3��˵�������������ӣ���3����������������ñ����ӣ�
��𣺽⣺��1���ɺ�ˮ��Ϊ�����Լ�PHֵԽ��ԽС���ٽ��ȫ�����к�ˮ��pH���α仯��PHֵ�ֿ�ʼ��Ϳ��������𰸣�
��2����ˮ��pH=8.1-8.3��˵���Լ��ԣ����������������ӣ�
��3���ⶨ��ˮ��Na2SO4������������Ҫ֪����ˮ���������ٸ������Ȼ�����Ӧ���ɳ�����������������Ƶ�������
�ʴ�Ϊ����1��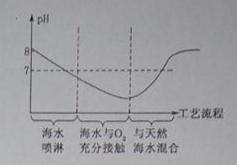
��2������������
��3�����⺣ˮ������BaCl2��
��𣺽⣺��1���ɺ�ˮ��Ϊ�����Լ�PHֵԽ��ԽС���ٽ��ȫ�����к�ˮ��pH���α仯��PHֵ�ֿ�ʼ��Ϳ��������𰸣�
��2����ˮ��pH=8.1-8.3��˵���Լ��ԣ����������������ӣ�
��3���ⶨ��ˮ��Na2SO4������������Ҫ֪����ˮ���������ٸ������Ȼ�����Ӧ���ɳ�����������������Ƶ�������
�ʴ�Ϊ����1��

��2������������
��3�����⺣ˮ������BaCl2��

��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�
�����Ŀ