��Ŀ����
 ʵ������һƿŨ���ᣬƿ�ϱ�ǩ��ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣮
ʵ������һƿŨ���ᣬƿ�ϱ�ǩ��ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣮��1������100g7.3% ��ϡ���ᣬ��Ҫ��Ũ�����������
20g
20g
����2�����ø����úõ�ϡ����ⶨ�����ֽ�����ɵĺϽ�ijɷ֣�ȡ20g�úϽ��ĩ��֮ǡ����ȫ��Ӧ����ˣ�������Һ����ʧ����������ϴ�ӡ�����Ϊ13.5g���ڿ����г�����մ��������õ���ɫ�����ĩ������ɫ��ĩ��������ϡ�����ϣ���ĩ��ȫ�ܽ⣬�õ���ɫ��Һ��
�ټ���Ͻ����������ᷴӦ�Ľ�����Ԫ�ط���ΪM ���ڻ������и�Ԫ��Ϊ+2 �ۣ���������ѧ��Ӧ�Ļ�ѧ����ʽΪ��
M+2HCl=MCl2+H2��
M+2HCl=MCl2+H2��
���ڸúϽ�����Ϊ
Cu��Zn
Cu��Zn
��������Ӧ�����Һ��ֻ��һ�����ʣ���Ӧ��������Һ�м���ˮ29.7g�����ʱ��Һ�����ʵ����������Ƕ��٣�
��������1��������Һϡ��ʱ��������������㣮
��2���ٸ��ݽ����ܺ��ᷴӦ�����κ�������������д����ʽ��
�ڸ�����Ϣ��������ϴ�ӡ�����Ϊ13.5g���ڿ����г�����մ��������õ���ɫ�����ĩ������ɫ��ĩ��������ϡ�����ϣ���ĩ��ȫ�ܽ⣬�õ���ɫ��Һ���Ƴ���������ͭ������M�����������������ʵ���������M�����ԭ�����������ж�Ԫ�����࣮
�۸��ݸ��ݻ�ѧ����ʽ�������ʵ����������������غ㶨�ɼ��㷴Ӧ����Һ���������ٸ�����Һ���������������ļ��㹫ʽ������������
��2���ٸ��ݽ����ܺ��ᷴӦ�����κ�������������д����ʽ��
�ڸ�����Ϣ��������ϴ�ӡ�����Ϊ13.5g���ڿ����г�����մ��������õ���ɫ�����ĩ������ɫ��ĩ��������ϡ�����ϣ���ĩ��ȫ�ܽ⣬�õ���ɫ��Һ���Ƴ���������ͭ������M�����������������ʵ���������M�����ԭ�����������ж�Ԫ�����࣮
�۸��ݸ��ݻ�ѧ����ʽ�������ʵ����������������غ㶨�ɼ��㷴Ӧ����Һ���������ٸ�����Һ���������������ļ��㹫ʽ������������
����⣺��1���⣺����Ҫ��Ũ�����������Ϊm����m��36.5%=lOOg��7.3%�����m=20g��
��2���ٽ������ᷴӦ�����κ�ˮ���ڻ�������MԪ��Ϊ+2�ۣ��������Ȼ���Ļ�ѧʽΪMCl2�����Է���ʽΪ��M+2HCl=MCl2+H2����
�ڸ�����Ϣ��������ϴ�ӡ�����Ϊ13.5g���ڿ����г�����մ��������õ���ɫ�����ĩ������ɫ��ĩ��������ϡ�����ϣ���ĩ��ȫ�ܽ⣬�õ���ɫ��Һ���Ƴ���������ͭ��
��M�����ԭ������ΪR��20g-13.5g=6.5g���Ȼ��������Ϊ100g��7.3%=7.3g����
M+2HCl=MCl2+H2��
R 73
6.5g 7.3g
=
���R=65������MΪп��
���跴Ӧ�����Ȼ�п������ΪY����������������ΪZ��
Zn+2HCl=ZnCl2+H2��
73 136 2
7.3g Y Z
=
�����Y=13.6g
=
�����Z=0.2g
��Ӧ�����Һ����ΪlOOg+6.5g-0.2g+29.7g=l36.0g����Ӧ����Һ��������������Ϊ
��100%=10%��
�𣺷�Ӧ����Һ��������������Ϊ10%��
�ʴ�Ϊ����1��20g����2����M+2HCl=MCl2+H2������Cu��Zn����10%��
��2���ٽ������ᷴӦ�����κ�ˮ���ڻ�������MԪ��Ϊ+2�ۣ��������Ȼ���Ļ�ѧʽΪMCl2�����Է���ʽΪ��M+2HCl=MCl2+H2����
�ڸ�����Ϣ��������ϴ�ӡ�����Ϊ13.5g���ڿ����г�����մ��������õ���ɫ�����ĩ������ɫ��ĩ��������ϡ�����ϣ���ĩ��ȫ�ܽ⣬�õ���ɫ��Һ���Ƴ���������ͭ��
��M�����ԭ������ΪR��20g-13.5g=6.5g���Ȼ��������Ϊ100g��7.3%=7.3g����
M+2HCl=MCl2+H2��
R 73
6.5g 7.3g
| R |
| 73 |
| 6.5g |
| 7.3g |
���R=65������MΪп��
���跴Ӧ�����Ȼ�п������ΪY����������������ΪZ��
Zn+2HCl=ZnCl2+H2��
73 136 2
7.3g Y Z
| 73 |
| 136 |
| 7.3g |
| Y |
| 73 |
| 2 |
| 7.3g |
| Z |
��Ӧ�����Һ����ΪlOOg+6.5g-0.2g+29.7g=l36.0g����Ӧ����Һ��������������Ϊ
| 13.6g |
| 136.0g |
�𣺷�Ӧ����Һ��������������Ϊ10%��
�ʴ�Ϊ����1��20g����2����M+2HCl=MCl2+H2������Cu��Zn����10%��
��������ѧ�����ǻ�ѧ������ȵ�֮һ���ر��ǹ��ڻ�ѧʽ�ļ��㡢������Һ�ļ��㡢���ڻ�ѧ����ʽ������ʽ����Һ���������������ϵļ��㣬�ڿ����г��ֵ�Ƶ����ߣ�

��ϰ��ϵ�д�
�����Ŀ
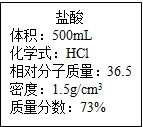 ʵ������һƿŨ���ᣬƿ�ϱ�ǩ������ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣺
ʵ������һƿŨ���ᣬƿ�ϱ�ǩ������ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣺ ʵ������һƿŨ���ᣬƿ�ϱ�ǩ��ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣮
ʵ������һƿŨ���ᣬƿ�ϱ�ǩ��ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣮ ʵ������һƿŨ���ᣬƿ�ϱ�ǩ�IJ���������ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣺
ʵ������һƿŨ���ᣬƿ�ϱ�ǩ�IJ���������ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣺ ʵ������һƿŨ���ᣬƿ�ϱ�ǩ��ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣮
ʵ������һƿŨ���ᣬƿ�ϱ�ǩ��ͼ��ʾ��������ݱ�ǩ���ṩ�����ݽ���������⣮