��Ŀ����
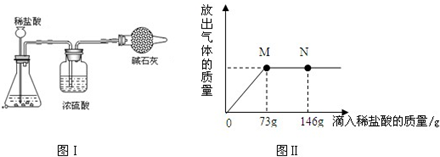
��ҵ�ϡ����ϡ��Ƽ�ƵĴ�����Ʒ�г����������Ȼ��ƣ�����Ա��ÿ�����������Ĵ����Ʒ��Ҫ���м�⣮������ֳɷֵĺ�����Ͷ���г�����ʵ������ͼ����ʾװ����Ͻ��вⶨ������װ�����������ã���װ�þ���������ȫ����ʯ���������ƺ��������ƵĻ�����ȡ22.3g����Ʒ��Aװ���У���������εμ����ʵ���������Ϊ10%��ϡ���ᣬ�ų�����������������μ�ϡ�����������ϵ������ͼ����ʾ��
���������ش����⣺
��1�����μ�ϡ������ͼ����N��ʱ���ձ�����Һ��������ǣ�д��ѧʽ��
��2�����μ���73gϡ����ʱ���ų������������Ϊ
��3�����μ���73gϡ����ʱ����M��ʱ�����ձ���Ϊ��������Һ����ͨ������������к����ʵ�����������
��4��С����Ϊ��ͼ��װ��������ⶨ������������������ƣ�������Ϊ

���������ش����⣺
��1�����μ�ϡ������ͼ����N��ʱ���ձ�����Һ��������ǣ�д��ѧʽ��
NaCl��HCl
NaCl��HCl
����2�����μ���73gϡ����ʱ���ų������������Ϊ
4.4
4.4
g����3�����μ���73gϡ����ʱ����M��ʱ�����ձ���Ϊ��������Һ����ͨ������������к����ʵ�����������
��4��С����Ϊ��ͼ��װ��������ⶨ������������������ƣ�������Ϊ
ϡ�����ӷ����Ȼ�������ᱻCװ������
ϡ�����ӷ����Ȼ�������ᱻCװ������
���ᵼ�½��ƫ��
ƫ��
���ƫ����ƫС��������������ͼ���֪������ϡ����73gʱ����Ʒ�е�̼���ƺ�ϡ����ǡ����ȫ��Ӧ����ʱ��Һ���Ȼ�����Һ�������ԣ�֮���ټ�ϡ������Һ�ͻ�����ԣ�Ȼ��ɸ���̼���ƺ�ϡ������ȫ��Ӧʱ���ĵ�������������㷴Ӧ��̼���ơ����ɶ�����̼���Ȼ��Ƶ����������������ݿɼ������Һ�����ʵ�����������װ��I����������ʹ�����ᷴӦʱ�����������ܻӷ����Ȼ������壬���Իᵼ�²����Ľ������ȷ���ݴ˷������ɣ�
����⣺��1����ͼ���֪��N��ʱ���ӵ�ϡ�����Ѿ����������Դ�ʱ��Һ�����ԣ�pHС��7��������Һ�е������Ƿ�Ӧ���ɵ��Ȼ��ƺ��������е��Ȼ��⣮
��2���贿����Ʒ��̼���Ƶ�����Ϊx�����ɶ�����̼������Ϊy���Ȼ��Ƶ�����z
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 117 44
x 73g��10% z y
=
=
=
��ã�x=10.6g��y=4.4g��z=11.7g
��3�����μ���73gϡ����ʱ����Һ�����ʵ���������Ϊ��
��100%=25.7%��
��4��ͼ��װ��������ⶨ������������������ƣ���Ϊϡ����Ҳ�ܻӷ����Ȼ������壬�Ȼ��������ܱ�Cװ�����յ��²����Ľ��������
�ʴ�Ϊ����1��NaCl��HCl����2��4.4����3��25.7%����4��ϡ�����ӷ����Ȼ�������ᱻCװ�����գ�ƫ��
��2���贿����Ʒ��̼���Ƶ�����Ϊx�����ɶ�����̼������Ϊy���Ȼ��Ƶ�����z
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 117 44
x 73g��10% z y
| 106 |
| x |
| 73 |
| 73g��10% |
| 117 |
| z |
| 44 |
| y |
��ã�x=10.6g��y=4.4g��z=11.7g
��3�����μ���73gϡ����ʱ����Һ�����ʵ���������Ϊ��
| 22.3g-10.6g+11.7g |
| 73g+22.3g-4.4g |
��4��ͼ��װ��������ⶨ������������������ƣ���Ϊϡ����Ҳ�ܻӷ����Ȼ������壬�Ȼ��������ܱ�Cװ�����յ��²����Ľ��������
�ʴ�Ϊ����1��NaCl��HCl����2��4.4����3��25.7%����4��ϡ�����ӷ����Ȼ�������ᱻCװ�����գ�ƫ��
������������Ҫ����ѧ������ȫ��Ӧ����ʶ�����û�ѧ����ʽ���м��������������Ĺؼ�������ͼ���жϷ�Ӧ��״����

��ϰ��ϵ�д�
�����Ŀ