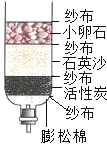
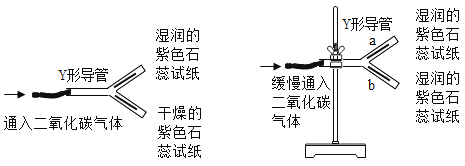
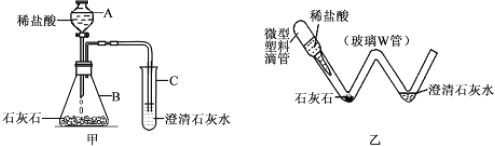
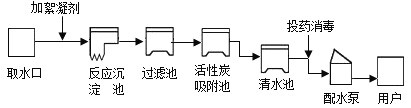
��Ŀ����
����Ŀ����������������ȫ��������LED��ʾ����Һ�����Ӳ�Ʒʹ��LED������LED��һ�ַ�������ܣ�����ʱ��___��ת��Ϊ___�ܵĹ�̬�뵼��������������Ԫ�ط���λGa���㷺��������뵼����ϣ���Ԫ�ص�ԭ�ӽṹʾ��ͼ����ͼ��ʾ��

��1����Ԫ�ص�������X=____����ԭ������������Ϊ_______��
��2����֪�����صij������ϼ�Ϊ+3������Ԫ�ص������ﻯѧʽΪ_____��
��3�������������������ƶ����м���������ʣ�������������ϡ���ᷢ���кͷ�Ӧ�Ļ�ѧ����ʽΪ_____________��
���𰸡��� �� 31 3 Ga2O3 2Ga(OH)3 ��3H2SO4��Ga2(SO4)3 ��3H2O
��������
��������������ȫ��������LED��ʾ����Һ�����Ӳ�Ʒʹ��LED������LED��һ�ַ�������ܣ�����ʱ��������ת��Ϊ���ܵĹ�̬�뵼��������
��1����Ԫ�ص�������=�����������X=2+8+18+3=31����ԭ������������Ϊ3��
��2����֪�����صij������ϼ�Ϊ+3������Ԫ�ص�����������Ԫ��Ϊ-2�ۣ���������Ļ�ѧʽΪGa2O3��
��3�������������������ƶ����м���������ʣ�������������ϡ���ᷢ���кͷ�Ӧ���������غ�ˮ�Ļ�ѧ����ʽΪ��2Ga(OH)3 ��3H2SO4��Ga2(SO4)3 ��3H2O��