��Ŀ����
 ��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ��pHֵ�仯�����ͼ��ʾ�����ƶ�X�Ļ�ѧʽΪ
��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ��pHֵ�仯�����ͼ��ʾ�����ƶ�X�Ļ�ѧʽΪNaOH
NaOH
��| ��Һ | A | B | C | D |
| X | ���� | ���� | �������� | �������� |
| Y | ˮ | �������� | ���� | ˮ |
C
C
����д��ţ�����2��д���÷�Ӧ�Ļ�ѧ����ʽ
NaOH+HCl=NaCl+H2O
NaOH+HCl=NaCl+H2O
����3����Y��Һ�е���ʯ����Һ�������ϵ���X��Һ��ʯ����ɫ�ı仯��
��ɫ��Ϊ��ɫ���ٱ�Ϊ��ɫ
��ɫ��Ϊ��ɫ���ٱ�Ϊ��ɫ
����4��д��a�㴦��Һ�����ʵĻ�ѧʽ��
NaOH��NaCl
NaOH��NaCl
����������1������pHֵ�仯���ͼ��������Һ���ȱ仯���ص㣬�����ṩ��������жԱȣ��ж���ȷ����ϣ�
��2��д��������ϵ����ʷ�����Ӧ�Ļ�ѧ����ʽ��
��3���������ָʾ��ʯ���ڱ仯��������ɫ�仯��
��4������ͼ����д��a�㴦��Һ�����ʵĻ�ѧʽ��
��2��д��������ϵ����ʷ�����Ӧ�Ļ�ѧ����ʽ��
��3���������ָʾ��ʯ���ڱ仯��������ɫ�仯��
��4������ͼ����д��a�㴦��Һ�����ʵĻ�ѧʽ��
����⣺��1����pHֵ�仯���ͼ����X�ļ�����Һ�����������ߣ���С��7������7�ٵ�����7��˵���μӵ���ҺΪ������Һ��ԭ��ҺYΪ������Һ����ѡC����ʼʱY��Һ��pH��7��һ�������ᣬ�μ�X��Һʱ�������ҺpH��С���ֱ��pH��7��ֻ���Ǽ�pH��7����Һ������NaOH��Һ��
��2��������������Ʒ�Ӧ�������Ȼ��ƺ�ˮ���ʴ�Ϊ��NaOH+HCl=NaCl+H2O��
��3���������еμ�ʯ�ʯ��ʺ�ɫ�������������ƵIJ��ϼ��룬��Һ���Լ�С��������ʱ��ʯ�����ɫ�����������ƹ�����Һ�ʼ��ԣ�ʯ�����ɫ���ʴ�Ϊ����ɫ��Ϊ��ɫ���ٱ�Ϊ��ɫ��
��4��a�㴦��ҺΪ���ԣ������ʵĻ�ѧʽNaOH��NaCl��
�ʴ�Ϊ��NaOH����1��C����2��NaOH+HCl=NaCl+H2O��
��3����ɫ��Ϊ��ɫ���ٱ�Ϊ��ɫ����4��NaOH��NaCl
��2��������������Ʒ�Ӧ�������Ȼ��ƺ�ˮ���ʴ�Ϊ��NaOH+HCl=NaCl+H2O��
��3���������еμ�ʯ�ʯ��ʺ�ɫ�������������ƵIJ��ϼ��룬��Һ���Լ�С��������ʱ��ʯ�����ɫ�����������ƹ�����Һ�ʼ��ԣ�ʯ�����ɫ���ʴ�Ϊ����ɫ��Ϊ��ɫ���ٱ�Ϊ��ɫ��
��4��a�㴦��ҺΪ���ԣ������ʵĻ�ѧʽNaOH��NaCl��
�ʴ�Ϊ��NaOH����1��C����2��NaOH+HCl=NaCl+H2O��
��3����ɫ��Ϊ��ɫ���ٱ�Ϊ��ɫ����4��NaOH��NaCl
������ʯ����һ�ֳ��õ����ָʾ���������죬�����������������Һ����ɫ��Ϊ��ɫ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��4�֣���X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ��pH�仯�����ͼ��ʾ��
| ��Һ | A | B | C | D |
| X | ���� | �������� | ���� | �������� |
| Y | ˮ | ˮ | �������� | ���� |
(2)д���÷�Ӧ�Ļ�ѧ����ʽΪ ��
(3)����X��Һ�IJ��ϵ��룬���ձ�����Һ���¶ȱ仯�� ��
(4)��Y��Һ�е���ʯ���Լ��������ϵ���X��Һ��ʯ��ı仯�� ��
(5)��X��Yǡ����ȫ��Ӧʱ����Һ�е������� ����Ҫ������Һ�л�ô������壬Ӧ�ò��� ��
 ��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ
��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ
��pH�仯�����ͼ��ʾ��
| ��Һ | A | B | C | D |
| X | ���� | �������� | ���� | �������� |
| Y | ˮ | ˮ | �������� | ���� |
��2��д���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3������X��Һ�IJ��ϵ��룬���ձ�����Һ���¶ȱ仯��______��
��4����Y��Һ�е���ʯ���Լ��������ϵ���X��Һ��ʯ��ı仯��______��
��5����X��Yǡ����ȫ��Ӧʱ����Һ�е�������______����Ҫ������Һ�л�ô������壬Ӧ�ò���______��
��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ
��pH�仯�����ͼ��ʾ��
��1�������з������ֱ仯������� ����д��ţ��������� ��
��2��д���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3������X��Һ�IJ��ϵ��룬���ձ�����Һ���¶ȱ仯�� ��
��4����Y��Һ�е���ʯ���Լ��������ϵ���X��Һ��ʯ��ı仯�� ��
��5����X��Yǡ����ȫ��Ӧʱ����Һ�е������� ����Ҫ������Һ�л�ô������壬Ӧ�ò��� ��
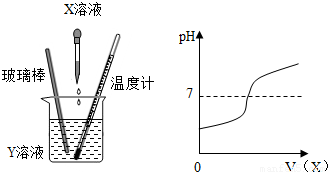
��pH�仯�����ͼ��ʾ��
| ��Һ | A | B | C | D |
| X | ���� | �������� | ���� | �������� |
| Y | ˮ | ˮ | �������� | ���� |
��2��д���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3������X��Һ�IJ��ϵ��룬���ձ�����Һ���¶ȱ仯�� ��
��4����Y��Һ�е���ʯ���Լ��������ϵ���X��Һ��ʯ��ı仯�� ��
��5����X��Yǡ����ȫ��Ӧʱ����Һ�е������� ����Ҫ������Һ�л�ô������壬Ӧ�ò��� ��

 ��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ
��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ