��Ŀ����
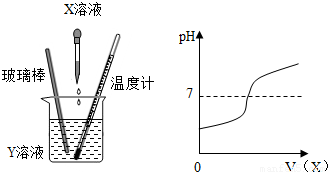
��4�֣���X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ��pH�仯�����ͼ��ʾ��
| ��Һ | A | B | C | D |
| X | ���� | �������� | ���� | �������� |
| Y | ˮ | ˮ | �������� | ���� |
(2)д���÷�Ӧ�Ļ�ѧ����ʽΪ ��
(3)����X��Һ�IJ��ϵ��룬���ձ�����Һ���¶ȱ仯�� ��
(4)��Y��Һ�е���ʯ���Լ��������ϵ���X��Һ��ʯ��ı仯�� ��
(5)��X��Yǡ����ȫ��Ӧʱ����Һ�е������� ����Ҫ������Һ�л�ô������壬Ӧ�ò��� ��
��1��D δ����X��Һʱ��Y��Һ������
��2����NaOH+HCl=NaCl+H2O
(3)�����ߺ�
��4�����ɺ�����ٱ���
��5����NaCl �����ܼ�
���������������1����ͼʾ��֪����Һ��PH��С��7����Ϊ����7���ʱ����з������ֱ仯�������D��������δ����X��Һʱ��Y��Һ�����ԣ�����X�ĵμӣ���Һ��Ϊ���ԣ�
(2)�÷�Ӧ�Ļ�ѧ����ʽΪNaOH+HCl=NaCl+H2O��
(3)����X��Һ�IJ��ϵ��룬���ձ�����Һ���¶ȱ仯�������ߺͣ���Ϊ������������Ʒ�Ӧ�ų������������������ƹ�������Һ���¶Ƚ����ͣ�
(4)��Y��Һ�е���ʯ���Լ��������ϵ���X��Һ��ʯ��ı仯���ɺ�����ٱ�����
(5)��X��Yǡ����ȫ��Ӧʱ����Һ�е�������NaCl����Ҫ������Һ�л�ô������壬Ӧ�ò��������ܼ��ķ�����
���㣺��Һ��PH�����ָʾ������ѧ��Ӧ�е������仯��������
������PH����7����Һ�Ǽ��Եģ�С��7�������Եģ�����7�������Եġ�
��ɫʯ����Һ�����ָʾ���������������ʱ��ɫ�������������ʱ���ɫ��
�Ȼ��Ƶ��ܽ�������¶ȱ仯�����ӣ���������Ȳ���һ����������ܼ��ķ������Ȼ�����Һ�еõ��Ȼ��ƾ��塣

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� ��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ
��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ
��pH�仯�����ͼ��ʾ��
| ��Һ | A | B | C | D |
| X | ���� | �������� | ���� | �������� |
| Y | ˮ | ˮ | �������� | ���� |
��2��д���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3������X��Һ�IJ��ϵ��룬���ձ�����Һ���¶ȱ仯��______��
��4����Y��Һ�е���ʯ���Լ��������ϵ���X��Һ��ʯ��ı仯��______��
��5����X��Yǡ����ȫ��Ӧʱ����Һ�е�������______����Ҫ������Һ�л�ô������壬Ӧ�ò���______��
��pH�仯�����ͼ��ʾ��
| ��Һ | A | B | C | D |
| X | ���� | �������� | ���� | �������� |
| Y | ˮ | ˮ | �������� | ���� |
��2��д���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3������X��Һ�IJ��ϵ��룬���ձ�����Һ���¶ȱ仯�� ��
��4����Y��Һ�е���ʯ���Լ��������ϵ���X��Һ��ʯ��ı仯�� ��
��5����X��Yǡ����ȫ��Ӧʱ����Һ�е������� ����Ҫ������Һ�л�ô������壬Ӧ�ò��� ��
 ��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ
��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ ��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ��pHֵ�仯�����ͼ��ʾ�����ƶ�X�Ļ�ѧʽΪ
��X��Һ����Y��Һ�У��ڵμӹ����У�Y��Һ��pHֵ�仯�����ͼ��ʾ�����ƶ�X�Ļ�ѧʽΪ