��Ŀ����
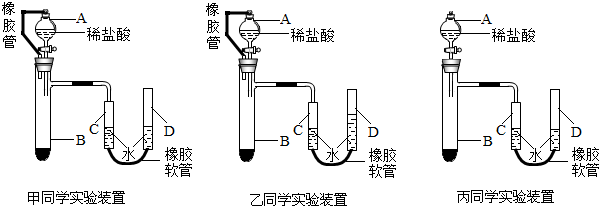
�ס��ҡ�����λͬѧ�������Լ��ֱ����ʵ�飬��ǡ����ȫ��Ӧ���й�ʵ���������±���| �����Լ������� | ��Ӧ��������Һ���� | |
| �� | CaO���̣�a1g��10%����b1g | c1g |
| �� | Ca��OH��2���̣�a2g��10%����b2g | c2g |
| �� | CaCO3���̣�a3g��10%����b3g | c3g |
�ֽ��ס��ҡ�����λͬѧ��Ӧ�����õ���Һȫ������һ�������ڣ��Ƶô���Һ����Ϊ158.1g������
��1���μӷ�Ӧ��̼��ƹ������������д��������̣�
��2����Ӧ��������Һ�����ʵ�����������
��3����Ӧ�����й�����ˮ��������
��������1����������Ӧ��ֻ�б����������������̼�������Ӧǰ���ձ��е����ʼ��ٵ�����Ϊ������̼������������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼�����������̼��Ƶ�������
��2����������Ӧ�Ļ�ѧ����ʽд�������ᷢ����������Ӧ�вμӷ�Ӧ�����������ɵ��Ȼ��Ƶ�������ֵһ�����ɸ����������������Ȼ��Ƶ��������ٸ���
��100%���������Ӧ��������Һ�����ʵ�����������
��3����Ӧ��������Һ�а����Ȼ��ơ�ԭϡ�����е�ˮ�ͷ�Ӧ���ɵ�ˮ���÷�Ӧ��������Һ������-ԭϡ�����е�ˮ������-�Ȼ��Ƶ��������������Ӧ����ˮ��������
��2����������Ӧ�Ļ�ѧ����ʽд�������ᷢ����������Ӧ�вμӷ�Ӧ�����������ɵ��Ȼ��Ƶ�������ֵһ�����ɸ����������������Ȼ��Ƶ��������ٸ���
| �Ȼ��Ƶ����� |
| ��Ӧ������Һ������ |
��3����Ӧ��������Һ�а����Ȼ��ơ�ԭϡ�����е�ˮ�ͷ�Ӧ���ɵ�ˮ���÷�Ӧ��������Һ������-ԭϡ�����е�ˮ������-�Ȼ��Ƶ��������������Ӧ����ˮ��������
����⣺��1����Ӧ������CO2������Ϊ��16.5g+146g-158.1g=4.4g
��μӷ�Ӧ��̼��ƹ�������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
=
��x=10g
�𣺲μӷ�Ӧ��̼��ƹ��������Ϊ10g��
��2���������Ȼ��Ƶ�����Ϊy
CaO+2HCl=CaCl2+H2O
Ca��OH��2+2HCl=CaCl2+2H2O
CaCO3+2HCl=CaCl2+H2O+CO2��
73 111
146g��10% y
=14.6g
=
��y=22.2g
��Ӧ��������Һ�����ʵ�����������
��100%=14.0%��
�ʴ�Ϊ��14.0
��3��158.1g-22.2g-��146g-14.6g��=4.5g��
�ʴ�Ϊ��4.5
��μӷ�Ӧ��̼��ƹ�������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
| 100 |
| 44 |
| x |
| 4.4g |
�𣺲μӷ�Ӧ��̼��ƹ��������Ϊ10g��
��2���������Ȼ��Ƶ�����Ϊy
CaO+2HCl=CaCl2+H2O
Ca��OH��2+2HCl=CaCl2+2H2O
CaCO3+2HCl=CaCl2+H2O+CO2��
73 111
146g��10% y
=14.6g
| 73 |
| 111 |
| 14.6g |
| y |
��Ӧ��������Һ�����ʵ�����������
| 22.2g |
| 158.1g |
�ʴ�Ϊ��14.0
��3��158.1g-22.2g-��146g-14.6g��=4.5g��
�ʴ�Ϊ��4.5
������������Ŀ�Ƚ��ѣ������ǵڶ�С�⣬�����Ǹ���ijһ����ѧ����ʽ���м��㣬�����ܽ��ij������ѧ��Ӧ����������Ĺ�ͬ�㣬Ȼ����һ����ѧ����ʽΪ�������м��㣮

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
�ס��ҡ�����λͬѧ�������Լ��ֱ����ʵ�飬��ǡ����ȫ��Ӧ�������Լ����������±�
��֪a1+a2+a3=23.04g��b1+b2+b3=189.8g���ֽ��ס��ҡ�����ͬѧ������Һȫ������һ�������ڣ��Ƶ���ҺΪ206.16g������˻����Һ��������������������ȷ��0.1%��
| �Լ������� | ��Ӧ��������Һ���� | ||
| �� | CaO���̣�a1g | 10%����b1g | C1 |
| �� | Ca��OH��2���̣�a2g | 10%����b2g | C2 |
| �� | CaCO3���̣�a3g | 10%����b3g | C3 |