��Ŀ����
����Ŀ��ˮ��һ����Ҫ����Դ������ˮ��Դ��������
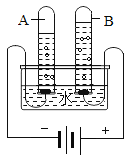
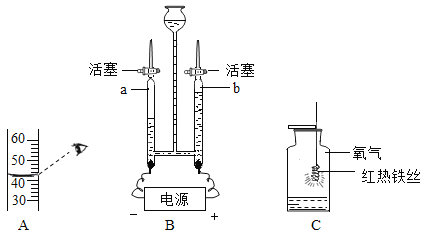
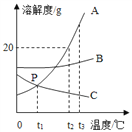
(1)���ˮ��ʵ����ͼ��ʾ���ռ����������Թ���_____(����A������B��)��д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________��
(2)ˮ����������ʷ�Ӧ����������ˮ���ҷ�Ӧ���ų��������ȣ������������ơ�д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________��
(3)�����У����dz�����__________�ķ������Ƚ���ˮ��Ӳ�ȣ���ɱ��������
(4)һЩ��������£�����ˮ�м���Ca(ClO)2 ����ɱ��������,������Ԫ�صĻ��ϼ���________��
���𰸡�B 2H2O ![]() 2H2��+O2�� CaO+H2O=Ca��OH��2 ��� +1
2H2��+O2�� CaO+H2O=Ca��OH��2 ��� +1
��������
��1�����ˮʱ������������ԼΪ���������2��������B������������ˮ��ͨ�������£��ֽ�������������������Ӧ�Ļ�ѧ����ʽ�ǣ�2H2O ![]() 2H2��+O2����
2H2��+O2����
���B��2H2O ![]() 2H2��+O2����
2H2��+O2����
��2�������ƺ�ˮ��Ӧ�����������ƣ��÷�Ӧ�Ļ�ѧ����ʽΪCaO+H2O=Ca��OH��2��
���CaO+H2O=Ca��OH��2��
��3�������У����dz�������еķ������Ƚ���ˮ��Ӳ�ȣ���ɱ��������
������
��4����Ca��ClO��2�и�Ԫ����+2�ۣ���Ԫ����-2�ۣ���Ԫ�صĻ��ϼ�Ϊx����+2+[x+��-2��] ��2=0��x=+1��
���+1��

 ��У����ϵ�д�
��У����ϵ�д�