��Ŀ����
��10�֣�ʵ���ҳ�����ͼA��B��Cװ����ȡ���壬�ش�������⣺
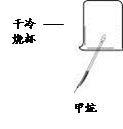
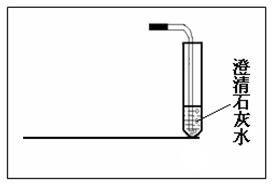
��1��ͼ�Тٵ�����������________�� ���Ȣ�ʱӦ����_________��
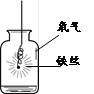
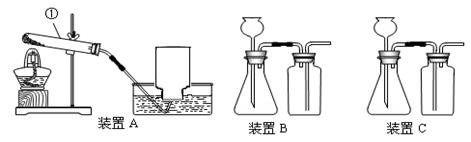
��2��С����Aװ����ȡ���ռ���������Ӧ�Ļ�ѧ����ʽΪ____________________��
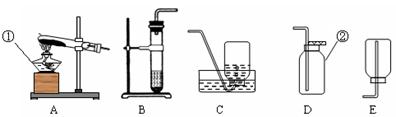
��3��ʵ������ȡ����Ӧ��ѡ�õ�װ����_____ ����װ����ĸ��ţ���������_____����������ţ���
�ٳ������ܷ�����Ӧ �ڷ�Ӧ�����Ǽ��� ���ܶȱȿ���С ���ܶȱȿ����� ��������ˮ
д����Ӧ�Ļ�ѧ����ʽ_____________________________��
��4��С��ͬѧ���������ͼ��ʾװ����ȡO2����ʵ��װ�õ��ŵ���___________________��

��ʵ��ǰ��Ը�װ�ý��������Լ�飬�����ǣ��رջ���������ƿ�м�ˮ��û����ĩ�ˣ�����ë����ס��ƿһ��ʱ�䣬���ɿ���ë���������ǣ���ʵ����̹�__________________����װ�����������á�

��1��ͼ�Тٵ�����������________�� ���Ȣ�ʱӦ����_________��
��2��С����Aװ����ȡ���ռ���������Ӧ�Ļ�ѧ����ʽΪ____________________��
��3��ʵ������ȡ����Ӧ��ѡ�õ�װ����_____ ����װ����ĸ��ţ���������_____����������ţ���
�ٳ������ܷ�����Ӧ �ڷ�Ӧ�����Ǽ��� ���ܶȱȿ���С ���ܶȱȿ����� ��������ˮ
д����Ӧ�Ļ�ѧ����ʽ_____________________________��
��4��С��ͬѧ���������ͼ��ʾװ����ȡO2����ʵ��װ�õ��ŵ���___________________��

��ʵ��ǰ��Ը�װ�ý��������Լ�飬�����ǣ��رջ���������ƿ�м�ˮ��û����ĩ�ˣ�����ë����ס��ƿһ��ʱ�䣬���ɿ���ë���������ǣ���ʵ����̹�__________________����װ�����������á�
����10�֣���1���Թܣ�Ԥ�ȡ�
��2��2KClO3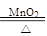 2KCl��3O2��
2KCl��3O2��
��3��B �٢ۻ�٢ۢ� Zn+H2SO4=ZnSO4+H2��
��4���ɿ��Ʒ�Ӧ���ʡ� ���ܿ�������ð�����ɿ���ë������Һ�������γ�һ��ˮ����
��2��2KClO3
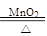 2KCl��3O2��
2KCl��3O2�� ��3��B �٢ۻ�٢ۢ� Zn+H2SO4=ZnSO4+H2��
��4���ɿ��Ʒ�Ӧ���ʡ� ���ܿ�������ð�����ɿ���ë������Һ�������γ�һ��ˮ����
���⿼������й�ʵ������ȡ�����֪ʶ������װ�õ�ѡ��Ҫ����Ӧ���״̬�ͷ�Ӧ��������Ӧ��Ϊ������Ҫ���ȣ�����װ��A,��Ӧ���Һ��ϲ���Ҫ���ȾͿ�����B��Cװ�á�Bװ���������ռ��ܶȱȿ���С�����壬Cװ���������ռ��ܶȱȿ���������塣

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ