题目内容
研究物质的性质和变化是认识物质的重要手段。请根据反应事实写出下列反应的化学方程式,并回答有关问题。
(1)细铁丝在氧气中燃烧____________________。
(2)红磷在空气中燃烧___________________。
(3)有水参加的分解反应______________________。
(4)教育部发出号召,自10月26日起,全国中小学生开展长跑活动。小王同学长跑后,感觉腿部酸痛,因为剧烈运动后血液中产生了较多乳酸(C3H6O3),使人肌肉酸痛,经过一段时间放松,由于乳酸与吸入的氧气反应生成二氧化碳和水,酸痛感消失。该反应的化学方程式为__________________________,属于__________(选填缓慢、剧烈)氧化反应。
(5)下示意图形象地表示了某化学反应前后分子的变化。其中 表示氧原子、
表示氧原子、 表示碳原子,反应的方程式为__________________________两种反应的化学计量系数(用符号表示)为____________________该示意图说明了化学反应的实质是___________________________
表示碳原子,反应的方程式为__________________________两种反应的化学计量系数(用符号表示)为____________________该示意图说明了化学反应的实质是___________________________
(1)细铁丝在氧气中燃烧____________________。
(2)红磷在空气中燃烧___________________。
(3)有水参加的分解反应______________________。
(4)教育部发出号召,自10月26日起,全国中小学生开展长跑活动。小王同学长跑后,感觉腿部酸痛,因为剧烈运动后血液中产生了较多乳酸(C3H6O3),使人肌肉酸痛,经过一段时间放松,由于乳酸与吸入的氧气反应生成二氧化碳和水,酸痛感消失。该反应的化学方程式为__________________________,属于__________(选填缓慢、剧烈)氧化反应。
(5)下示意图形象地表示了某化学反应前后分子的变化。其中
 表示氧原子、
表示氧原子、 表示碳原子,反应的方程式为__________________________两种反应的化学计量系数(用符号表示)为____________________该示意图说明了化学反应的实质是___________________________
表示碳原子,反应的方程式为__________________________两种反应的化学计量系数(用符号表示)为____________________该示意图说明了化学反应的实质是___________________________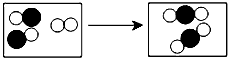
(1)3Fe+2O2 Fe3O4
Fe3O4
(2)4P+5O2 2P2O5
2P2O5
(3)2H2O 2H2↑+O2↑
2H2↑+O2↑
(4)C3H6O3+3O2==3CO2+3H2O;缓慢
(5)2CO+O2 2CO2;
2CO2;  =2:1;分子分裂成原子,原子重新组合成新的分子
=2:1;分子分裂成原子,原子重新组合成新的分子
 Fe3O4
Fe3O4(2)4P+5O2
 2P2O5
2P2O5 (3)2H2O
 2H2↑+O2↑
2H2↑+O2↑(4)C3H6O3+3O2==3CO2+3H2O;缓慢
(5)2CO+O2
 2CO2;
2CO2;  =2:1;分子分裂成原子,原子重新组合成新的分子
=2:1;分子分裂成原子,原子重新组合成新的分子
练习册系列答案
 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案
相关题目
 表示氧原子、
表示氧原子、 表示碳原子,则请回
表示碳原子,则请回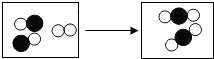
 表示氧原子、
表示氧原子、 表示碳原子,则该反应的化学方程式为:
表示碳原子,则该反应的化学方程式为: