��Ŀ����
����Ŀ������Ʒ�㷺Ӧ���������������С�
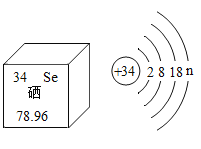
��1����������_______(����ԭ������������������������)���ɡ�
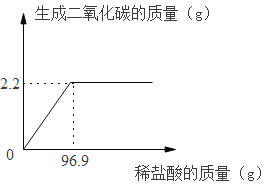
��2����������Ʒ�к�̼����ߵ���______(ѡ����ĸ)��
A����� B���� C��ͨ��
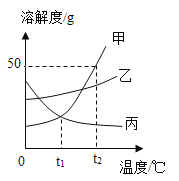
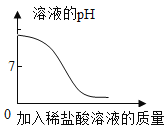
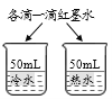
��3���������⣬����______�仯(ѡ��������������ѧ��)����ͼ��̽�������ڲ�ͬ�����·��������ʵ�飬��������ʴ������________(ѡ����ĸ) ��
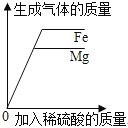
��4���������ᴿ��õ��Ĵ���Fe2O3������ij�ֹ�ҵ����(��Ҫ�ɷ�ΪFeO��Fe2O3)���䷴Ӧԭ��Ϊ:![]() ���ֽ���̿������Fe2O3��Ͼ��ȣ�������ԭ����ַ�Ӧ�������1.1kg��CO2������_____kg��FeO��
���ֽ���̿������Fe2O3��Ͼ��ȣ�������ԭ����ַ�Ӧ�������1.1kg��CO2������_____kg��FeO��
���𰸡�ԭ�� ���� ��ѧ B 7.2
��������
��1�������ڽ������ʣ���������ԭ�ӹ��ɣ�
��2���ڲ���֡���������ͨ����������Ʒ�к�̼����ߵ���������
��3���������⣬�����������ɣ����ڻ�ѧ�仯��������������������ˮ�Ӵ�����ͼ��̽�������ڲ�ͬ�����·��������ʵ�飬��������ʴ������B��
��4��������FeO������Ϊx��
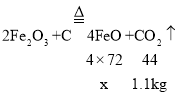
![]() ��
��
x=7.2kg�������1.1kg��CO2������7.2kg��FeO��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ѧ��ȤС���ͬѧ��ѧϰ�εĻ�ѧ����ʱ����������ʵ��:��һ������NaHCO3��Һ�м���һ������ϡHCl,��Ӧ�Ļ�ѧ����ʽ��:![]() ,����ȫ��Ӧ��(���跴Ӧ����������ȫ���ݳ�)���Է�Ӧ����Һ�����ʳɷֽ�������̽����(��ѡ�Լ���Ʒ: pH��ֽ��þƬ��ϡ���ᡢAgNO3��Һ������ͭ��ĩ��ʯ����Һ)����ɲ��룬�������������:
,����ȫ��Ӧ��(���跴Ӧ����������ȫ���ݳ�)���Է�Ӧ����Һ�����ʳɷֽ�������̽����(��ѡ�Լ���Ʒ: pH��ֽ��þƬ��ϡ���ᡢAgNO3��Һ������ͭ��ĩ��ʯ����Һ)����ɲ��룬�������������:
(���������)
����1:_____________������2: NaCl�� NaHCO3������3: NaCl�� HCl������4: NaCl�� NaHCO3��HCl��
ͬѧ�Ǿ�������һ����Ϊ���� 4��������������:_______________��
(ʵ��̽��)
ʵ����� | ʵ������ | ʵ����� |
��1��ȡ��Ӧ����Һ������ϡ���� | �����ݲ��� | ����_________������ |
��2��ȡ��Ӧ����Һ������pH��ֽ�� | ��ֽ��ɫ�� ���ձ�ɫ����pH<7 | ����3���� |
��3��ȡ��Ӧ����Һ����������ͭ��ĩ | _______________________ | ����3������д����Ӧ�Ļ�ѧ����ʽ:______________ |
��4��ȡ��Ӧ����Һ���μ�AgNO3��Һ | ������ɫ���� | ����3���� |
(�ó�����)����3��ȷ��
(�����뷴˼)��ʦ��ͬѧ�ǵ�̽�����̸����˿϶���ͬʱָ��̽���д�����һ�����ԵĴ���ԭ����___________________��
(�ܽ����)����������ʵ����Ʒ���㻹��ʲô��ͬ�ķ���ȷ�ϲ���3����ȷ�ġ���д��һ��ʵ�鷽��:___________________��
����Ŀ�����Ʊ�����ʵ��̽������Ҫ����������ʵ����������ﵽʵ��Ŀ�ĵ��ǣ�������
|
|
|
|
A��̽������þ�������ǿ�� | B��̽��������̼��ˮ�Ƿ�����Ӧ | C��̽����ͬ�ܼ��������ܽ��Դ�С | D��̽���¶ȶԷ����˶�������Ӱ�� |
A. AB. BC. CD. D