��Ŀ����
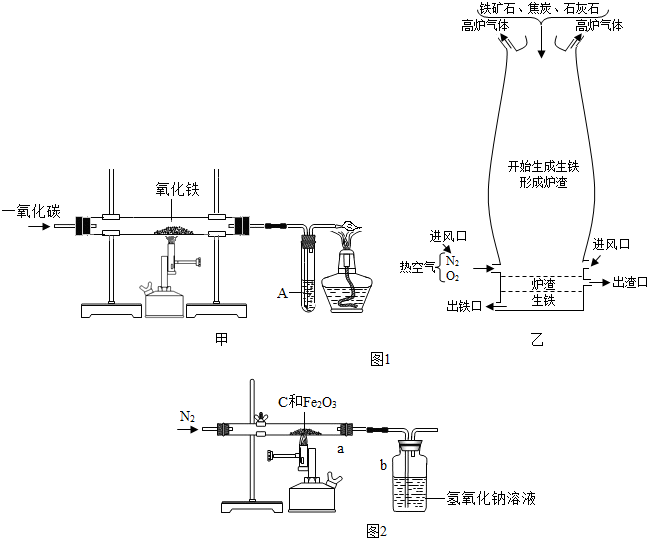
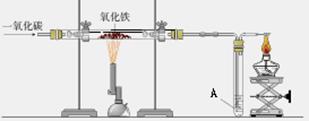
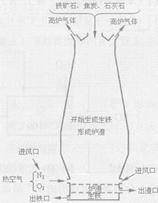
��ͼ������һ����̼����������Ӧװ�õ�ʾ��ͼ����ͼ�Ǹ�¯����ʾ��ͼ����ش��������⣺��A��Ϊ����ʯ��ˮ��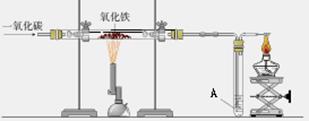

�� ��
��1����ͼʵ������У���Ҫ���е��� ������� ���� ��ͨ��CO
��2����ͼ��Ӳ�ʲ����������Ӧ�������� �� ���� ���۲쵽A�����ֵ������� �� ���� �����з����Ļ�ѧ��Ӧ����ʽ�� �� ���� ��
��3����ͼ��ʵ��Ҫ��װ��ĩ��a��ȼ��һյ�ƾ��ƣ��������� �� ���� �����з����Ļ�ѧ��Ӧ����ʽ�� �� ���� ��
��4����ͼ�и�¯����ʱ��̿�������� �� �� �� ��
��5��Ϊʲô��ͼ�г����ڵ���¯�����ڣ� ��
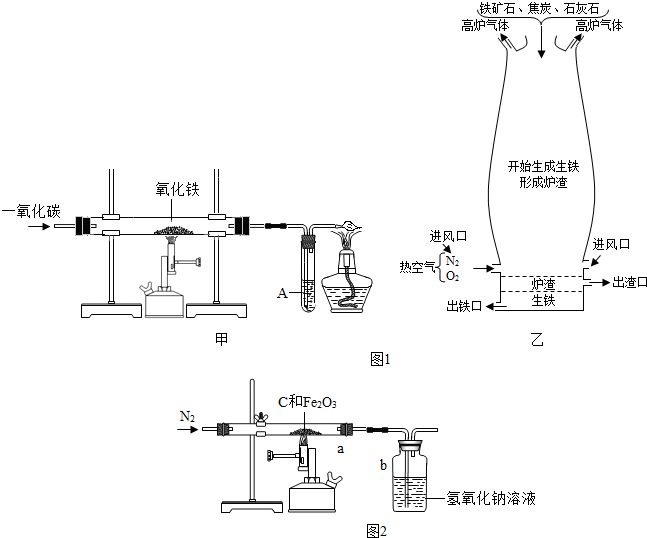
��6����ѧ��ȤС���ͬѧΪ�˲ⶨij������ʯ��������������������
ijͬѧȡһ�������ij�������������ľ̿�ۻ�Ϻ�����ͼ��ʾװ���Ժ����IJ�������ⶨ������������Һ�������ն�����̼���壩��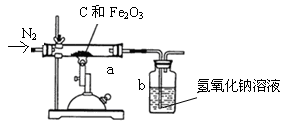
��ʵ���г���ͨ�����ĵ���������ǰ����ͨ��һ��ʱ�䣬�������� �� ���� ��
��ֹͣ����ǰ�Ƿ���Ҫ�ȶϿ�a��b�����Ӵ��Է�ֹ������Ϊʲô��
�� ���� ��
��7���Լ��㣺�ҹ�����ʯ��Դ�ȽϷḻ��ij��������1000 t ��������80% �ij�����ʯ�������Ͽ�����������96% �������������Ƕ��٣�������һλС����
��1����
��2����ɫ��ĩ��ɺ�ɫ�������ʯ��ˮ����� Ca(OH)2 +CO2==CaCO3 ��+ H2O
��3������β������ʹCO�������CO2 2CO+O2 2CO2
2CO2
��4������CO��ȼ�շ������¯�� ��5�������ܶȱ�¯����
��6���ų�����������ʵ����� ����Ҫ�����ϵ�ͨ�뵪����b�е�����������Һ���ᵹ����a�У��������ɣ� ��7��583.3t
���������������1����ͼʵ������У���Ҫ���е��Ǣ�ͨ��CO����װ���ڵĿ����ž���
��2����ͼ��Ӳ�ʲ����������Ӧ�������Ǻ�ɫ��ĩ��ɺ�ɫ���۲쵽A�����ֵ������dz����ʯ��ˮ����ǣ����з����Ļ�ѧ��Ӧ����ʽ��Ca(OH)2 +CO2==CaCO3 ��+ H2O��
��3����ͼ��ʵ��Ҫ��װ��ĩ��a��ȼ��һյ�ƾ��ƣ��������ǽ���β������ʹCO�������CO2�����з����Ļ�ѧ��Ӧ����ʽ��2CO+O2 2CO2��
2CO2��
��4����ͼ�и�¯����ʱ��̿�������Dz���CO��ȼ�շ��ȣ����¯�£�
��5����Ϊ�����ܶȱ�¯����������ͼ�г����ڵ���¯�����ڣ�
��6����ʵ���г���ͨ�����ĵ���������ǰ����ͨ��һ��ʱ�䣬���������ų�����������ʵ����
��ֹͣ����ǰ����Ҫ�ȶϿ�a��b�����Ӵ��Է�ֹ��������Ϊ���ϵ�ͨ�뵪������b�е�����������Һ���ᵹ����a�С�
��7���裺��1000 t ��������80% �ij�����ʯ�������Ͽ�����������96% ��������������X��1000t��80%��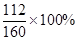 =X��96%��X=583.3t��
=X��96%��X=583.3t��
���㣺��¯������ԭ������ѧ����ʽ����д��һ����̼�����ʺ���;����������Ԫ�ص�����������
��������¯����������һ����̼�Ļ�ԭ�ԣ�����������ԭΪ����
��д��ѧ����ʽҪ��ѭ����ʵ�������غ㶨������ԭ��ע�⻯ѧʽҪ��ȷ����Ҫ���Ƿ�Ӧ������������߳������š�
ijԪ�ص���������= ��
��