��Ŀ����
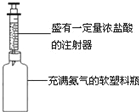
����Ŀ����Լ��ˮ������ˮ��Դ������ÿλ����Ӧ������������Σ����������˽��һЩ�й�ˮ��֪ʶ����ش��������⣺��ͼ1��ijͬѧ�����ļ���ˮ��
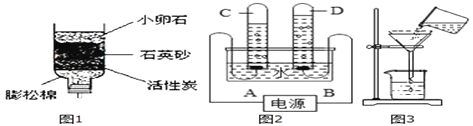
(1)С��ʯ��ʯӢɰ��������_______������̿����_________���ã�
(2)��������Ӳˮ�Խ���������Ҫ����ˮ��Ӳ�ȣ��ɲ�ȡ_______�ķ���������������__________����Ӳˮ����ˮ��
(3)������Ϊ���ڽ�Լ��ˮ����________(����ĸ)��
A�����ֹ�ˮ��ͷ B����ϴ�ˡ�ϴ�µ�ˮ����Ͱ C����ϴ�»�ϴһ˫˿�࣮
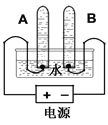
ͼ2��ʾ����ʵ���ҵ��ˮ��װ��
(4)��ֱͨ�����D�ܲ�����������_______��A�缫Ϊ��Դ��_________����
(5)�ɸ�ʵ��ɵó��Ľ�����___________________��
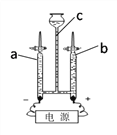
Ϊ�˲ⶨһ������ˮ����ɳ��������ijͬѧ�������ͼ3��ʾ��ʵ�������
(6)��ʵ�黹Ӧ���������������__________����������_____________��
(7)��������������ʵ�黹����������
��_______________________��___________________________��
���𰸡� ����(��������) ���� ��� ����ˮ AB ���� �� ˮ������Ԫ�غ���Ԫ����ɵ� ������ ���� ��ֽ��Ե����©����Ե ©�����¶�δ���ձ��ڱ�
����������������������⿼����ˮ�ľ�����
��1��С��ʯ��ʯӢɰ�������ǹ��ˣ�����̿������������ɫ�غ���ζ��
��2��������п��Խ���ˮ��Ӳ������ˮ�м������ˮ���裬�����������ĭ�϶�����ˮ�������������ĭ������Ӳˮ����
��3��A�����ֹ�ˮ��ͷ�����Խ�Լ��ˮ������ȷ��
B����ϴ�ˡ�ϴ�µ�ˮ����Ͱ�����Խ�Լ��������ȷ��
C����ϴ�»�ϴһ˫˿�࣬���ˮ�˷ѣ��ʴ���
��4���۲�ͼ��֪���Թ�D�����������Ϊ�������Թ�C��������϶�Ϊ��������A�缫Ϊ��Դ�ĸ�����
��5���ɸ�ʵ��ɵó��Ľ�����ˮ������Ԫ�غ���Ԫ����ɵģ�
��6����ͼʾ��֪�ò����ǹ��ˣ�������Ҫ�ò�����������ͼ��û�в���������������������������
��7����ͼʾ��֪����ͼ���ڵ����Դ����ǣ���ֽ�ı�Ե����©���ı�Ե��Ӧ�õ���©����Ե��©�����¶�û�н����ձ����ڱڣ�Ӧ�����ձ��ڱ���

����Ŀ�������100 gijƷ�ư����IJ���Ӫ���ɷ֡�
������ | ������ | ���� | ��֬ | �� | ά����C |
1 016 kJ | 5.1 g | 11.3 g | 19 g | 4.6 mg | 3.3 mg |
(1)ʳ��ijɷ���Ҫ��������Ӫ�������ϱ���Ϊ�����ṩ��Ҫ��������________��
(2)����ð����е���Ԫ�����Ȼ��Ƶ���ʽ��������100 g�ð��������Ȼ��Ƶ�������________��