��Ŀ����
��Լ��Դ�ͱ��������Ѿ���Ϊ���ǵĻ������ߡ����ܼ��š�������������̬�н�������������Щ��Ĺ����ص㡣���᳧��������������ǣ��Ѻ������ȼ�գ����ɶ�������������������ڸ��ºʹ���������������������������������ˮ�����������ᡣд�����������ˮ������������Ļ�ѧ����ʽ
ij������Ʒ�к������������ƣ������ⶨ��̼���Ƶ��������������ú�����������ij����ˮ��������ʵ�飺
��ʵ��ԭ����Na2CO3+H2SO4= Na2SO4 + H2O + CO2��ͨ��ʵ��ⶨ��Ӧ�����Ķ�����̼���������������ԭ��Ʒ��̼���Ƶ�����,�������̼��������Ʒ�е�����������
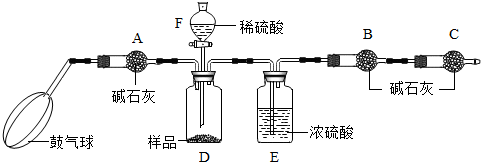
��ʵ��װ�á�

��ʵ�鲽�衷
����ͼ����װ�ã���B��C�⣩����������ҩƷ��
�ڳ�������¼B��������m1����������ʱע����B�����ˡ���
�۰������������������Լ1���ӡ�
��������B��C��
�ݴ�Һ©��F�Ļ�������ϡ������ټ���D�кرջ�����
�ް�����������Լ1���ӡ�
�߳�������¼B��������m2����������ʱע����B�����˼�E�Ҷ˵ij��ڡ���
����㡣
����֪��ʯ�ҵ���Ҫ�ɷ����������ƺ��������ƣ�������A�������ǣ� ������ʹ�ⶨ���ƫ��
��2�� ���ܻ��ܣ���ϡ�������ϡ���ᣬ��Ϊ������� �ԣ���ʹ���̼���Ƶ��������� ����ƫ��ƫС�䣬��ͬ������ȥ�������C������̼���Ƶ�������������
��3��Eװ�õ�������
��4����ʵ���ܷ�ʡ�Ԣۡ�����������? �����ܻ��ܣ���ԭ��ֱ��� ��
��5������ȡ��Ʒ������Ϊ6g����Һ©��F��ʢ��5��ֻ������һ�����ʵij����ˮ���Ƶ�m1Ϊ51.20g��m2Ϊ53.40g����������������λС����
��1����Ʒ��̼���Ƶ���������Ϊ���٣�
��2��Dװ�������÷�Ӧ��������Һ���������������Ƕ��٣�
SO3+H2O=H2SO4 �� ���տ����еĶ�����̼��ˮ����ֹ����ʵ�顣
��2�����ܡ��ӷ��ԡ�ƫ��ƫ��3���������ɵĶ�����̼
��4�������ԣ���Ϊ��Ҫ�ÿ����϶�����̼����ֹ���ɵĶ�����̼�ܽ�ˮ��
��5��m������̼=m2��m1=2.20g��m̼����=5.30g ���ɵ� m̼����=7.1g
m̼��=4.9g m̼����Һ=4.9g ��5��=98g
Na2CO3%= /6��100%=88.33% Dװ����������Һ����������������Na2SO4%=(6-5.3+7.1��g/��98+6-2.2��g=7.66%
/6��100%=88.33% Dװ����������Һ����������������Na2SO4%=(6-5.3+7.1��g/��98+6-2.2��g=7.66%
����:ʵ��Ŀ�ģ��ⶨij������Ʒ����̼���Ƶ�����������ͨ��ʵ��ⶨ��Ӧ�����Ķ�����̼���������������ԭ��Ʒ��̼���Ƶ�����,�������̼��������Ʒ�е���������������ܶ������տ����еĶ�����̼��ˮ����ֹ����ʵ�顣��Dװ�������÷�Ӧ��������Һ��Ϊ��������Һ��������������Ʒ����һ���֣�������һ���֡��ؼ�����Һ��������Ҫע����ʵ����������̼�ݳ�Ҫ��ȥ��������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�