��Ŀ����
����������ҹ��ж����������������ص�����֮�У����ֵ�������ʱ��������£����������ѿ̲��ݻ���
ʮ�˴�Ҳ����̬�������������ͻ���ĵ�λ��ǿ���ˡ���ֽ�Լ��Դ�ͱ��������Ļ������ߡ���
I���������γɵ�ԭ��
��1������ָ����ϸ�ĸɳ����Ⱦ��ȵ������ڿ��У�ʹˮƽ�ܼ���С��10km�������ձ���ǵ��������γ�ԭ��������������⣬ú�����ͺͲ���ȼ��ʱ��________���ŷŵ��������γɸ�����Ҳ����Ҫԭ��֮һ��
II������������ɳɷ֣�
������ɳɷַdz����ӣ����к����ʶ�������ϸ�����PM2.5���ϣ���ǰ��֪����Ҫ�ɷ�Ϊ�����Ρ������Ρ���Ρ���̼�������ؽ����ȣ�
��2������PM2.5����С��������������������е�________���к����岢���������ʽӴ�������Ӧ�������������Ρ������Σ�
��3��Ǧ��Pb���������ϼۣ�+2����ͭ���dz������ؽ���������һ����ѧ����ʽ��ʾ���ߵĻ��˳��________��
��4�������Σ�������أ�����Σ����Ȼ�泥������ೣ���İ��ʣ���������ء��Ȼ�淋ķ�����ʲô�����������������ۣ���
III���١������ķ�����
��5���ı���Դ�ṹ�Ǽ�С������Ⱦ�ķ���֮һ��
Ϊ��һ��������Ⱦ�����ƿ������������������ļ�ȼú�糧��Ҫ���С�ú��������Ȼ���������̣���ʱÿ�꽫�����ŷ�7500t�Ķ����������壮
��д��������Ȼ��Ԫ����ɵ�ʵ�鷽�������������������ۣ���
����ú�е���Ԫ���Ե�����ƣ�������ú���������̣�ÿ������ú���3%��ú���ٶ֣�
�⣺��1��ú�����ͺͲ���ȼ��ʱ�����ɵ�С��0.1�Ŀ������ŷŵ��������γɸ��������γ�������Ҫԭ��֮һ��
�ʴ�Ϊ�����ɵ�С��0.1�Ŀ�����
��2��PM2.5�����������еĶ�����������������к����岢���������ʽӴ�������Ӧ�������������Ρ������Σ�
�ʴ�Ϊ����������
��3������Ǧ�Ľ�����Ա�ͭǿ����Ǧ�ܴ�����ͭ��Һ���û���ͭ������Ǧ���������Һ��Ӧʱһ����+2�ۣ���Ӧ�Ļ�ѧ����ʽPb+CuSO4=Cu+PbSO4��
�ʴ�Ϊ��Pb+CuSO4=Cu+PbSO4��
��4������ȡ����������������һ�ձ��У����������ռ���Һ���ȣ��ᵽ�̼�����ζ����笠����ӵ��������ȷ�Ӧ���ɰ����������Ȼ�泥�û����ζ��������أ�
�ʴ�Ϊ��ȡ����������������һ�ձ��У����������ռ���Һ���ȣ��ᵽ�̼�����ζ����笠����ӵ��������ȷ�Ӧ���ɰ����������Ȼ�泥�û����ζ��������أ�
��5������Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ�����ɶ�����̼��ˮ��ȡ�����ȼ���ڻ�����Ϸ����ϸ���IJ����������Կ�����ˮ�����ɣ�˵��������ˮ��������Ԫ�أ��ٽ���ȼ������ͨ������ʯ��ˮ�������а�ɫ�������ɣ�˵�����ɵ������Ƕ�����̼������̼Ԫ�أ�
�ʴ�Ϊ��ȡ�����ȼ���ڻ�����Ϸ����ϸ���IJ����������Կ�����ˮ�����ɣ�˵��������ˮ��������Ԫ�أ��ٽ���ȼ������ͨ������ʯ��ˮ�������а�ɫ�������ɣ�˵�����ɵ������Ƕ�����̼������̼Ԫ�أ�
����ÿ���ŷ�7500t�Ķ�������������Ҫ�������ΪX
S+O2 SO2
SO2
32 64
X 7500t
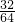 =
=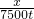
X=3750t
ÿ������ú���3%��ú��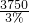 =125000t��
=125000t��
��ÿ������ú���3%��ú125000t��
�ʴ�Ϊ��125000t��
��������1��ú�����ͺͲ���ȼ��ʱ�����ɵ�С��0.1�Ŀ������ŷŵ��������γɸ��������γ�������Ҫԭ��֮һ��
��2��PM2.5�����������еĶ�����������������к����岢���������ʽӴ�������Ӧ�������������Ρ������Σ�
��3���û��ǿ�Ľ����ܽ�������Ľ�����������Һ���û��������ص㣬��Ƽ�������̵Ľ�����Ա���ǿ��ʵ�飬д����Ӧ�Ļ�ѧ����ʽ��
��4�����������Ӧ�����ɴ̼�����ζ�İ���������
��5������Ȼ������Ҫ�ɷ��Ǽ��飬���ݼ����ȼ���������������ijɷ֣�
�ڸ�����ȼ�����ɶ�������ķ���ʽ���㼴�ɣ�
���������⿼���֪ʶ��϶࣬�ѶȽϴ���Ҫͬѧ��ϸ�Ŀ��ǣ�
�ʴ�Ϊ�����ɵ�С��0.1�Ŀ�����
��2��PM2.5�����������еĶ�����������������к����岢���������ʽӴ�������Ӧ�������������Ρ������Σ�
�ʴ�Ϊ����������
��3������Ǧ�Ľ�����Ա�ͭǿ����Ǧ�ܴ�����ͭ��Һ���û���ͭ������Ǧ���������Һ��Ӧʱһ����+2�ۣ���Ӧ�Ļ�ѧ����ʽPb+CuSO4=Cu+PbSO4��
�ʴ�Ϊ��Pb+CuSO4=Cu+PbSO4��
��4������ȡ����������������һ�ձ��У����������ռ���Һ���ȣ��ᵽ�̼�����ζ����笠����ӵ��������ȷ�Ӧ���ɰ����������Ȼ�泥�û����ζ��������أ�
�ʴ�Ϊ��ȡ����������������һ�ձ��У����������ռ���Һ���ȣ��ᵽ�̼�����ζ����笠����ӵ��������ȷ�Ӧ���ɰ����������Ȼ�泥�û����ζ��������أ�
��5������Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ�����ɶ�����̼��ˮ��ȡ�����ȼ���ڻ�����Ϸ����ϸ���IJ����������Կ�����ˮ�����ɣ�˵��������ˮ��������Ԫ�أ��ٽ���ȼ������ͨ������ʯ��ˮ�������а�ɫ�������ɣ�˵�����ɵ������Ƕ�����̼������̼Ԫ�أ�
�ʴ�Ϊ��ȡ�����ȼ���ڻ�����Ϸ����ϸ���IJ����������Կ�����ˮ�����ɣ�˵��������ˮ��������Ԫ�أ��ٽ���ȼ������ͨ������ʯ��ˮ�������а�ɫ�������ɣ�˵�����ɵ������Ƕ�����̼������̼Ԫ�أ�
����ÿ���ŷ�7500t�Ķ�������������Ҫ�������ΪX
S+O2
 SO2
SO232 64
X 7500t
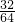 =
=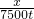
X=3750t
ÿ������ú���3%��ú��
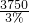 =125000t��
=125000t����ÿ������ú���3%��ú125000t��
�ʴ�Ϊ��125000t��
��������1��ú�����ͺͲ���ȼ��ʱ�����ɵ�С��0.1�Ŀ������ŷŵ��������γɸ��������γ�������Ҫԭ��֮һ��
��2��PM2.5�����������еĶ�����������������к����岢���������ʽӴ�������Ӧ�������������Ρ������Σ�
��3���û��ǿ�Ľ����ܽ�������Ľ�����������Һ���û��������ص㣬��Ƽ�������̵Ľ�����Ա���ǿ��ʵ�飬д����Ӧ�Ļ�ѧ����ʽ��
��4�����������Ӧ�����ɴ̼�����ζ�İ���������
��5������Ȼ������Ҫ�ɷ��Ǽ��飬���ݼ����ȼ���������������ijɷ֣�
�ڸ�����ȼ�����ɶ�������ķ���ʽ���㼴�ɣ�
���������⿼���֪ʶ��϶࣬�ѶȽϴ���Ҫͬѧ��ϸ�Ŀ��ǣ�

��ϰ��ϵ�д�
�����Ŀ